- #1
Lo.Lee.Ta.
- 217
- 0
I cannot for the life of me figure out where the O-O-H comes from that got added in the
3rd step!
Here's image of the reaction mechanism:
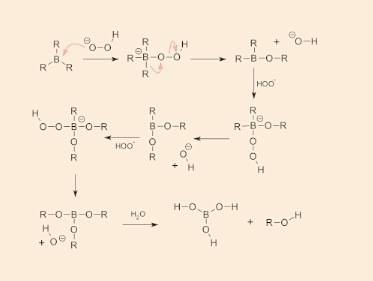
The H2O is not used until the last step and the H2O2 has already been used (except for the H+), so the other O that adds to the OH- to form O-O-H must be coming from the NaOH when it dissociates into Na+ and OH-...
But this would not make sense because we would have an OH- and an O- adding together, and that would be too many electrons!
Thank you so much! :)
3rd step!
Here's image of the reaction mechanism:
The H2O is not used until the last step and the H2O2 has already been used (except for the H+), so the other O that adds to the OH- to form O-O-H must be coming from the NaOH when it dissociates into Na+ and OH-...
But this would not make sense because we would have an OH- and an O- adding together, and that would be too many electrons!
Thank you so much! :)