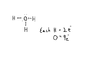- #1
krackers
- 72
- 0
[H3O]+ has an extra electron, so shouldn't it be negatively charged?
Similarly, [NCO]-, with (for simplicity sake) a ressonance structure of N=C=O means N needs an electron, and thus the whole molecule is short of one electron. Doesn't that mean it is positively charged?
Similarly, [NCO]-, with (for simplicity sake) a ressonance structure of N=C=O means N needs an electron, and thus the whole molecule is short of one electron. Doesn't that mean it is positively charged?