- #1
Hyla Brook
- 23
- 0
Dear all,
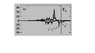
I found in many references the authors pointed out the eg and t2g band in the DOS graphics, and wrote in the text accordingly that it had eg symmetry (or t2g symmetry). How did they conclude that just from one Dos graphics (sometimes d projected)?
Best regards
I found in many references the authors pointed out the eg and t2g band in the DOS graphics, and wrote in the text accordingly that it had eg symmetry (or t2g symmetry). How did they conclude that just from one Dos graphics (sometimes d projected)?
Best regards