- #1
ProblemSets
- 22
- 0
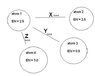
1. which letter shows a bond where electrons are being transferred?
2. which letter shoes a bond where electrons are equally shared?
3. which letter shows a bond that would cause each atom to become partially charged
4. which atom can be considered an anion?
5. which bond above would cause the compound to have the highest melting point?
answers: Y,X,Z,4,Y
are they correct??
thanks!
P.S. EN stands for electronegativity
2. which letter shoes a bond where electrons are equally shared?
3. which letter shows a bond that would cause each atom to become partially charged
4. which atom can be considered an anion?
5. which bond above would cause the compound to have the highest melting point?
answers: Y,X,Z,4,Y
are they correct??
thanks!
P.S. EN stands for electronegativity