- #1
katiecool
- 4
- 0
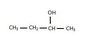
I am struggling to understand how to determine splitting patterns for molecules. For example, (see attached) I was trying to determine the splitting pattern for CH2 in the molecule. On the right i believe n is equal to 1 and on the right it is equal to 3, but i am not sure if this is correct. A doublet quartet doesn't make any sense to me and that is what i seem to conclude. The addition of the OH is really throwing me off. Also, on top of that how would i begin to determine which is farthest down field! Any help would be great!