- #1
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Equilibrium Stat Mech Vs. Kinetics
- Thread starter bolbteppa
- Start date
-
- Tags
- Equilibrium Kinetics Stat mech
In summary, the passage discusses the concept of subsystems in a closed system and the difficulty of obtaining an exact solution for their behavior due to the complex and intricate interactions with the rest of the system. It also introduces the ergodic hypothesis, which states that in a sufficiently long time, the phase trajectory of a subsystem will pass through each small volume of its phase space multiple times, allowing for the calculation of probabilities for the subsystem's state. The passage further explains this concept using the example of a single particle and its trajectory in phase space, and the extension to multiple particles.
Physics news on Phys.org
- #2
bolbteppa
- 309
- 41
Related to this point (functioning as something which might explain the above more deeply), what is the actual meaning of this passage from Landau vol. 5:
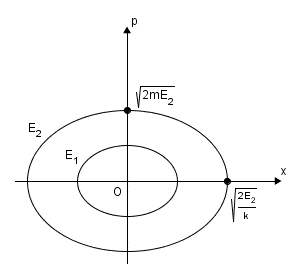
Again as an example, take [itex] H(q,p) = \tfrac{p^2}{2m}+\tfrac{k}{2}q^2 = E_0[/itex] as the Hamiltonian for a single particle, the trajectory of the particle, i.e. the set of all possible states, is an ellipse in (q,p) phase space.
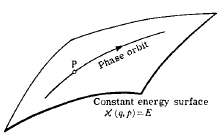
Extend it to n particles and we'll have a Cartesian product of n ellipses, or a big ellipsoid, visualized as
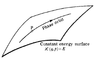
What in the world does that passage really mean, in terms of n harmonic oscillators respresented as a single curve in phase space? I can't make actually make any sense out of it.
Let us now consider a macroscopic body or system of bodies, and assume that the system is closed, i.e. does not interact with any other bodies. A part of the system, which is very small compared with the whole system but still macroscopic, may be imagined to be separated from the rest; clearly, when the number of particles in the whole system is sufficiently large, the number in a small part of it may still be very large. Such relatively small but still macroscopic parts will be called subsystems. A subsystem is again a mechanical system, but not a closed one; on the contrary, it interacts in various ways with the other parts of the system. Because of the very large number of degrees of freedom of the other parts, these interactions will be very complex and intricate. Thus the state of the subsystem considered will vary with time in a very complex and intricate manner.
An exact solution for the behaviour of the subsystem can be obtained only by solving the mechanical problem for the entire closed system, i.e. by setting up and solving all the differential equations of motion with given initial conditions, which, as already mentioned, is an impracticable task. Fortunately, it is just this very complicated manner of variation of the state of subsystems which, though rendering the methods of mechanics inapplicable, allows a different approach to the solution of the problem.
A fundamental feature of this approach is the fact that, because of the extreme complexity of the external interactions with the other parts of the system, during a sufficiently long time the subsystem considered will be many times in every possible state. This may be more precisely formulated as follows. Let dp dq denote some small "volume" of the phase space of the subsystem, corresponding to coordinates q, and momenta p, lying in short intervals dq, and dp,. We can say that, in a sufficiently long time T, the extremely intricate phase trajectory passes many times through each such volume of phase space. Let dt be the part of the total time T during which the subsystem was in the given volume of phase space dp dq. When the total time T increases indefinitely, the ratio dt/T tends to some limit w = lim dt/T.
This quantity may clearly be regarded as the probability that, if the subsystem is observed at an arbitrary instant, it will be found in the given volume of phase space dpdq.
Again as an example, take [itex] H(q,p) = \tfrac{p^2}{2m}+\tfrac{k}{2}q^2 = E_0[/itex] as the Hamiltonian for a single particle, the trajectory of the particle, i.e. the set of all possible states, is an ellipse in (q,p) phase space.
Extend it to n particles and we'll have a Cartesian product of n ellipses, or a big ellipsoid, visualized as
What in the world does that passage really mean, in terms of n harmonic oscillators respresented as a single curve in phase space? I can't make actually make any sense out of it.
- #3
TSC
- 39
- 1
This is the ergodic hypothesis.
1. What is the difference between Equilibrium Statistical Mechanics and Kinetics?
Equilibrium Statistical Mechanics and Kinetics are two branches of physical chemistry that study the behavior of molecules and atoms. The main difference between them is that Equilibrium Statistical Mechanics focuses on systems at thermodynamic equilibrium, while Kinetics studies the rates at which reactions occur.
2. How are the concepts of Equilibrium and Kinetics related?
Equilibrium and Kinetics are closely related because they both involve the study of chemical reactions and the behavior of molecules. Kinetics determines the rate at which reactions occur, while Equilibrium Statistical Mechanics looks at the distribution of molecules at equilibrium.
3. What are the main principles of Equilibrium Statistical Mechanics?
Equilibrium Statistical Mechanics is based on three main principles: the microscopic reversibility principle, the ergodic hypothesis, and the Boltzmann distribution. These principles help to explain the behavior of molecules in equilibrium and predict the macroscopic properties of a system.
4. How does Kinetics help us understand chemical reactions?
Kinetics helps us understand chemical reactions by studying the rates at which they occur. By measuring the rate of a reaction, we can determine the order of the reaction, the rate constant, and other important parameters that describe the reaction. This information can then be used to optimize reaction conditions or predict the outcome of a reaction.
5. What are some real-life applications of Equilibrium Statistical Mechanics and Kinetics?
Equilibrium Statistical Mechanics and Kinetics have numerous real-life applications, such as in the design of new materials, drug development, and environmental studies. Understanding the principles of these branches of chemistry can also aid in the production of more efficient and sustainable energy sources.
Similar threads
-
Science and Math Textbooks
- Replies
- 3
- Views
- 1K
-
Science and Math Textbooks
- Replies
- 10
- Views
- 1K
- Replies
- 2
- Views
- 1K
-
Classical Physics
- Replies
- 2
- Views
- 776
-
STEM Academic Advising
- Replies
- 6
- Views
- 834
- Replies
- 9
- Views
- 4K
- Replies
- 3
- Views
- 891
- Replies
- 8
- Views
- 2K
-
Mechanics
- Replies
- 16
- Views
- 2K
-
Chemistry
- Replies
- 3
- Views
- 1K
Share: