- #1
jrodatus
- 11
- 0
Hey, I came across this idea a couple of years ago. It's been a long time since I took introductory physics. But I'm curious as to whether this is feasible. If I'm crazy, just say so!
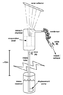
See attached image.
1. Intake tube, vacuum chamber and condenser are initially filled with water. Valve (A) remains open; valve (B) is closed. Vacuum chamber and condenser are raised to a height more than 10 meters above the intake reservoir.
2. Condenser is temporarily oriented such that its contents can drain into the chamber while the hydrostatic vacuum is forming. Water level in the intake tube settles at a height about 10 meters above the reservoir, balancing atmospheric pressure, while a vacuum is sustained in the chamber and condenser above that height. Valve (A) is closed.
3. At this point, the shelf inside the vacuum chamber has retained a significant amount of water from draining back down the intake tube. A solar collector focuses sunlight through the top of the glass container to slightly warm this water (>80˚F).
4. The warm water rapidly vaporizes in the presence of the vacuum. Water vapor diffuses into the condenser tube, condenses and accumulates at valve (B).
5. There must be enough space between the condenser and valve (B) to hold the same volume of water as does the evaporation shelf in the vacuum chamber. Once the transfer is complete, valve (B) is opened to discharge the distilled liquid. (Obviously there should be a separate valve on the chamber to let in atmosphere lest we blow the distilled water back inside).
6. In order to restart the process, both valves are opened while the displacement pump refills the vacuum chamber and condenser with water. Condenser is temporarily raised/oriented such that its contents do not flow out the open valve (B).
7. Once chamber and condenser are filled, valve (A) remains open and valve (B) is closed. Return to step 2.
It seems that the only energy supplied besides sunlight is that for refilling the vacuum chamber with water each time. Here's my attempt at estimating the work required per use.
Intake tube diameter: 1 cm
Intake tube length: 1000 cm
Intake tube volume: 785.4 cm^3 = .7854 L
Vacuum chamber volume: 2 L
Condenser volume: 1.5 L
:: Total volume of water displacement: .7854 + 2 + 1.5 = 4.2854 L
Mass of displacement: 4.2854 kg
Height of displacement: 1 m (guess)
:: Work done: 4.2854 kg * 9.81 m/s^2 * 1 m = 42.04 J
Evaporation shelf volume: 1 L
Energy input per liter distilled: ~42 J/L
Considering that it ordinarily takes a whopping 2,573,598 J to boil off a liter of water, that figure is pretty unbelievable. Maybe I need to account for the work to overcome atmospheric pressure in lifting the water? Hmm.
See attached image.
1. Intake tube, vacuum chamber and condenser are initially filled with water. Valve (A) remains open; valve (B) is closed. Vacuum chamber and condenser are raised to a height more than 10 meters above the intake reservoir.
2. Condenser is temporarily oriented such that its contents can drain into the chamber while the hydrostatic vacuum is forming. Water level in the intake tube settles at a height about 10 meters above the reservoir, balancing atmospheric pressure, while a vacuum is sustained in the chamber and condenser above that height. Valve (A) is closed.
3. At this point, the shelf inside the vacuum chamber has retained a significant amount of water from draining back down the intake tube. A solar collector focuses sunlight through the top of the glass container to slightly warm this water (>80˚F).
4. The warm water rapidly vaporizes in the presence of the vacuum. Water vapor diffuses into the condenser tube, condenses and accumulates at valve (B).
5. There must be enough space between the condenser and valve (B) to hold the same volume of water as does the evaporation shelf in the vacuum chamber. Once the transfer is complete, valve (B) is opened to discharge the distilled liquid. (Obviously there should be a separate valve on the chamber to let in atmosphere lest we blow the distilled water back inside).
6. In order to restart the process, both valves are opened while the displacement pump refills the vacuum chamber and condenser with water. Condenser is temporarily raised/oriented such that its contents do not flow out the open valve (B).
7. Once chamber and condenser are filled, valve (A) remains open and valve (B) is closed. Return to step 2.
It seems that the only energy supplied besides sunlight is that for refilling the vacuum chamber with water each time. Here's my attempt at estimating the work required per use.
Intake tube diameter: 1 cm
Intake tube length: 1000 cm
Intake tube volume: 785.4 cm^3 = .7854 L
Vacuum chamber volume: 2 L
Condenser volume: 1.5 L
:: Total volume of water displacement: .7854 + 2 + 1.5 = 4.2854 L
Mass of displacement: 4.2854 kg
Height of displacement: 1 m (guess)
:: Work done: 4.2854 kg * 9.81 m/s^2 * 1 m = 42.04 J
Evaporation shelf volume: 1 L
Energy input per liter distilled: ~42 J/L
Considering that it ordinarily takes a whopping 2,573,598 J to boil off a liter of water, that figure is pretty unbelievable. Maybe I need to account for the work to overcome atmospheric pressure in lifting the water? Hmm.
Attachments
Last edited:
 But what do I know?
But what do I know?