- #1
YoshiBeans
- 8
- 0
My question is regarding the reason why the Brownian ratchet fails to negate the second law.
The explanation that I have been told relates to the fact that the molecules in the second chamber would interact with the 'pawl' in a similar manner to those interacting with the paddle wheel, negating the overall useful rotation.
I have also been told that if T1 is higher than T2, then it will produce useful work, but that this complies with the laws by acting like a heat engine.
My question is: How can the same failure to extract useful work be explained if there was a vacuum in the second chamber? This would negate the temperature comparison, and would seem to allow the extraction of work from the first chamber.
I would appreciate any available illumination. Thank you.
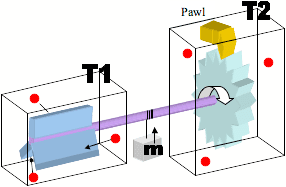
The explanation that I have been told relates to the fact that the molecules in the second chamber would interact with the 'pawl' in a similar manner to those interacting with the paddle wheel, negating the overall useful rotation.
I have also been told that if T1 is higher than T2, then it will produce useful work, but that this complies with the laws by acting like a heat engine.
My question is: How can the same failure to extract useful work be explained if there was a vacuum in the second chamber? This would negate the temperature comparison, and would seem to allow the extraction of work from the first chamber.
I would appreciate any available illumination. Thank you.