- #1
merbear
- 12
- 0
1. Homework Statement
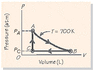
Two moles of an ideal gas are carried around the thermodynamic cycle shown in Fig. 18-29. The cycle consists of (1) an isothermal expansion A to B at a temperature of 700 K, with the pressure at A given by pA = 9 atm; (2) an isobaric compression B to C at PC = 4 atm; and (3) an isochoric pressure increase C A. What work is done by the gas per cycle? (See attached picture)
2. Homework Equations
pv=nRT
W=pdv
P=nRT/V
3. The Attempt at a Solution
W=nRT*ln(v2/v1)
For Pa:
9 atm= [2 mol*(.08205784 L*atm/k*mol)*700K]/V2
For Pb:
4 atm= [2 mol*(.08205784 L*atm/k*mol)*700K]/V1
V2/V1= 4/9
W=nRT(ln(4/9)) = 93.16 J (for A to B)
Here is where I got stuck. I can't seem to find the work for B to C without having variables in the answer. Once I can find the work from B to C I can add the work quantities to find the total (and ignore the value for C to B because the total work with no volume change is zero).
Any help in finding work from B to C would be appreciated.
Thank you
1. Homework Statement
2. Homework Equations
3. The Attempt at a Solution
Two moles of an ideal gas are carried around the thermodynamic cycle shown in Fig. 18-29. The cycle consists of (1) an isothermal expansion A to B at a temperature of 700 K, with the pressure at A given by pA = 9 atm; (2) an isobaric compression B to C at PC = 4 atm; and (3) an isochoric pressure increase C A. What work is done by the gas per cycle? (See attached picture)
2. Homework Equations
pv=nRT
W=pdv
P=nRT/V
3. The Attempt at a Solution
W=nRT*ln(v2/v1)
For Pa:
9 atm= [2 mol*(.08205784 L*atm/k*mol)*700K]/V2
For Pb:
4 atm= [2 mol*(.08205784 L*atm/k*mol)*700K]/V1
V2/V1= 4/9
W=nRT(ln(4/9)) = 93.16 J (for A to B)
Here is where I got stuck. I can't seem to find the work for B to C without having variables in the answer. Once I can find the work from B to C I can add the work quantities to find the total (and ignore the value for C to B because the total work with no volume change is zero).
Any help in finding work from B to C would be appreciated.
Thank you
1. Homework Statement
2. Homework Equations
3. The Attempt at a Solution