- #1
lapo3399
- 55
- 0
Hi,
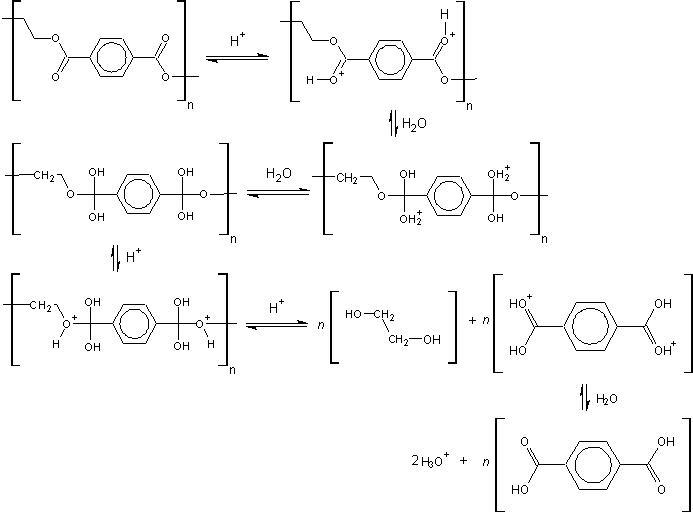
I am wondering whether the following drawing I made accurately represents the mechanism of the acid-catalyzed hydrolysis of polyethylene terephthalate. I am unsure as to whether both the ester bonds should break (as I have shown below), although I believe this is the case. Also, please notify me of any structural errors or errors in nomenclature/drawing/balancing (e.g. the final hydronium, which I assumed was 2H3O+ because of the 2 extra hydrogens leaving the terephthalic [sic] acid). Any other help would also be appreciated. (Click to make larger...)
Thanks!
I am wondering whether the following drawing I made accurately represents the mechanism of the acid-catalyzed hydrolysis of polyethylene terephthalate. I am unsure as to whether both the ester bonds should break (as I have shown below), although I believe this is the case. Also, please notify me of any structural errors or errors in nomenclature/drawing/balancing (e.g. the final hydronium, which I assumed was 2H3O+ because of the 2 extra hydrogens leaving the terephthalic [sic] acid). Any other help would also be appreciated. (Click to make larger...)
Thanks!