- #1
QMechanic
- 11
- 0
I got confused when in my book they went from one form of schrodinger equation to another. It doesn't make much sense to me algebraically, probably i have some lacks in complex numbers. Here are the equations:
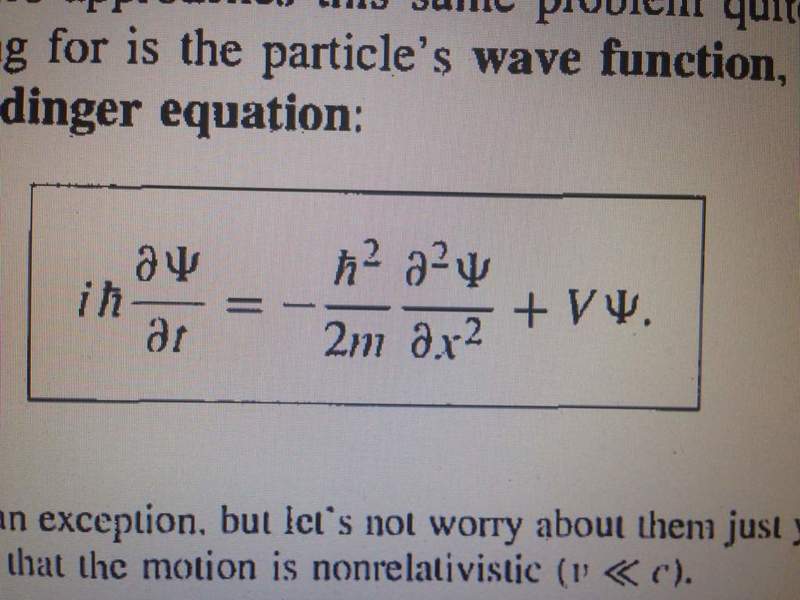
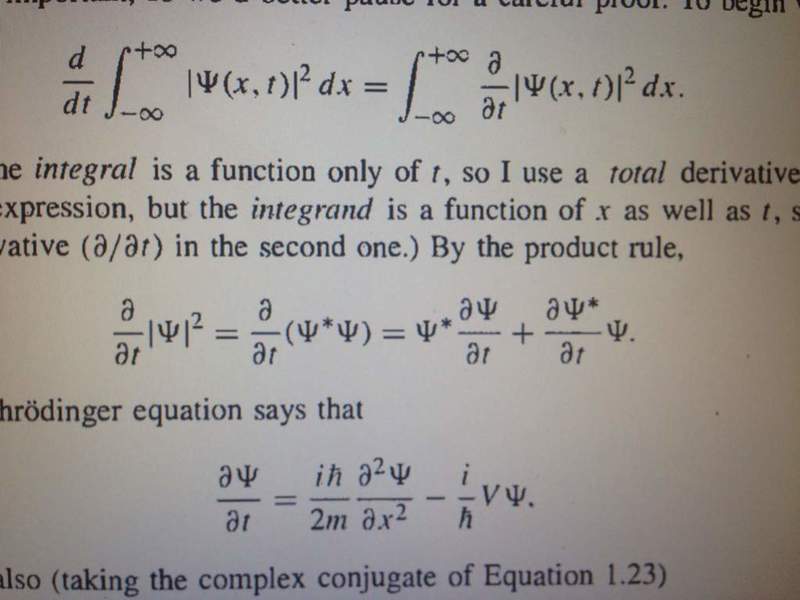
In the second one I think it's implied that above two equations give third and I am not sure how.
In the second one I think it's implied that above two equations give third and I am not sure how.