- #1
somasimple
Gold Member
- 766
- 5
Hi All,
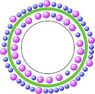
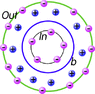
In the attached figure, I have drawn some "virtual" ions of two kind: blue are sodium, and violet for potassium (normally solvated with water).
There is more ions outside of my "vat" (bilipidic layer) than inside.
All ions are supposed as positive.
Since there is a difference in number of ions between the two sides, is the internal ions attracted (because outside is more positive than inside) or repulsed (because ions are positive on the two sides), or anything else?
In the attached figure, I have drawn some "virtual" ions of two kind: blue are sodium, and violet for potassium (normally solvated with water).
There is more ions outside of my "vat" (bilipidic layer) than inside.
All ions are supposed as positive.
Since there is a difference in number of ions between the two sides, is the internal ions attracted (because outside is more positive than inside) or repulsed (because ions are positive on the two sides), or anything else?