- #1
CrimpJiggler
- 149
- 1
Lets say I need to predict the number of infrared absorbance peaks that will appear on the IR spectrum of a given molecule. As an example, I'll use this molecule:
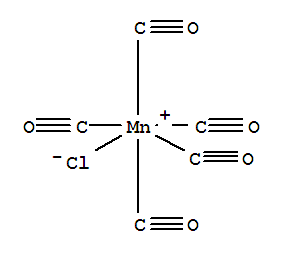
I can see that the point group of this molecule is C4v. I can look up this point groups character table:
http://img685.imageshack.us/img685/7360/charactertb.png
I'll be honest, I have no idea what this character table actually means but from what I remember, it tells me how many IR and raman absorbances the molecule has. If I'm not mistaken, the table says this molecule has 2 IR absorbances and 2 raman absorbances. How do I find out which of these are IR or raman active? In the past, I came across 2 methods involving irreducible representations and other stuff that I don't understand and I couldn't get either method to work. How do YOU do this? I'm looking for a good, solid algorithm that I can use to solve this problem for the majority of molecules that I come across.
I can see that the point group of this molecule is C4v. I can look up this point groups character table:
http://img685.imageshack.us/img685/7360/charactertb.png
I'll be honest, I have no idea what this character table actually means but from what I remember, it tells me how many IR and raman absorbances the molecule has. If I'm not mistaken, the table says this molecule has 2 IR absorbances and 2 raman absorbances. How do I find out which of these are IR or raman active? In the past, I came across 2 methods involving irreducible representations and other stuff that I don't understand and I couldn't get either method to work. How do YOU do this? I'm looking for a good, solid algorithm that I can use to solve this problem for the majority of molecules that I come across.
Last edited by a moderator: