- #1
Misr
- 385
- 0
hi ,world
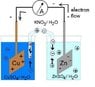
1-What is the importance of salt bridge?
According to my textbook :
1-connects between the two solutions without allowing a direct contact between them
So what is the problem of direct contact?
2-Neutralizes the charges which are formed in the solution of two half cells as a result of red-ox reactions in the zinc and copper half cells.
OK ,that doesn't make any sense
Still don't understand the function of salt bridge.
2- Why do we put salt in the salt bridge and why shouldn't its ions react with the ions present in the two half cells?
3-Sometimes the anode is negative like in galvanic cell,so I want a general definition for the anode in any cell.
4-What is meant by electrode potential?can you help me understand the concept of electric potential and potential difference because we always learn these stuff in a terrible way and i think they are very hard to understand.
5-Electromotive series "the arrangement of the standard potentials of the elements in a descending way with respect to the negative reduction potentials and ascending relative to the positive reduction potentials"
Oh I'm very confused about this.
does this mean "oxidation potential"?
thanks in advance.
1-What is the importance of salt bridge?
According to my textbook :
1-connects between the two solutions without allowing a direct contact between them
So what is the problem of direct contact?
2-Neutralizes the charges which are formed in the solution of two half cells as a result of red-ox reactions in the zinc and copper half cells.
OK ,that doesn't make any sense
Still don't understand the function of salt bridge.
2- Why do we put salt in the salt bridge and why shouldn't its ions react with the ions present in the two half cells?
3-Sometimes the anode is negative like in galvanic cell,so I want a general definition for the anode in any cell.
4-What is meant by electrode potential?can you help me understand the concept of electric potential and potential difference because we always learn these stuff in a terrible way and i think they are very hard to understand.
5-Electromotive series "the arrangement of the standard potentials of the elements in a descending way with respect to the negative reduction potentials and ascending relative to the positive reduction potentials"
Oh I'm very confused about this.
does this mean "oxidation potential"?
thanks in advance.