- #1
Vulgar
- 36
- 0
Hi,
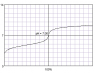
I was wondering why titration is a good method for determining concentrations. For instance to determine the concentraion of a weak acid HAc with a strong base NaOH,
NaOH+HAc --> NaAc+H20
Does this reaction have an equilibrium factor of inifinite or a very big one so that equivalent amounts of weak acid and base just neutralize each other?
Can u determine the concentration of a weak acid with a weak base? I don't think so because the total reaction doesn't have a very big equilibrium factor.
Kind regards
vulgar
I was wondering why titration is a good method for determining concentrations. For instance to determine the concentraion of a weak acid HAc with a strong base NaOH,
NaOH+HAc --> NaAc+H20
Does this reaction have an equilibrium factor of inifinite or a very big one so that equivalent amounts of weak acid and base just neutralize each other?
Can u determine the concentration of a weak acid with a weak base? I don't think so because the total reaction doesn't have a very big equilibrium factor.
Kind regards
vulgar