- #1
AlexHT
- 13
- 0
Hi,
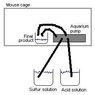
I am trying to do a experiment on artificially induced hibernation of mice. I am trying to send 80ppm of a hibernation inducting substance in the atmosphere of the mouse cage. The compound is H2S which is generally toxic but completely safe at these levels. H2S is gaseous at -60 degrees Celsius and above but can be dissolved in water.
I am looking for ideas and suggestions on how to setup a system that will outgaz 80ppm (but ideally tunable from 0-200ppm) into a isolated hermetic mouse cage.
I am trying to do a experiment on artificially induced hibernation of mice. I am trying to send 80ppm of a hibernation inducting substance in the atmosphere of the mouse cage. The compound is H2S which is generally toxic but completely safe at these levels. H2S is gaseous at -60 degrees Celsius and above but can be dissolved in water.
I am looking for ideas and suggestions on how to setup a system that will outgaz 80ppm (but ideally tunable from 0-200ppm) into a isolated hermetic mouse cage.