- #1
_Greg_
- 38
- 0
Hi folks
I'm currently in my last year of my chemical engineering degree and doing a design project on the manufacture of acrylic acid. I have the job of designing a solvent recovery column, where a mixture of Methylisobutylketone (MIBK) solvent (heavy key) and acrylic acid (light key) are to be separated.
To be honest with you guys I'v not had many good grades throughout my course and it's at the design stages where I suffer as a result, and I'v been stressing over this a lot, time is just passing me by and don't seem to be getting anywhere, so whould really appreciate some help.
So firstly I'll show you my feed composition:
Component-----------------Feed (kg/s) 60*C, 13kPa
Propene-----------------------0
Oxygen------------------------0
Nitrogen-----------------------0.0251
Carbon Dioxide-----------------0.0500
Water-------------------------0.5391
Acrylic Acid--------------------8.5548 (LK)
Acrolein-----------------------7.5365
Acetic Acid--------------------0.2035
MIBK--------------------------29.3277 (HK)
Total---------------------------46.2367
My Assumptions are:
· Key Components: MIBK and Acrylic Acid
· Pseudo-Binary mixture
· 100% recovery of Acrylic & Acetic Acid in bottoms
· 0% solvent & water in bottoms
· From the research I'v done, packing seems to be the way to go for this purpose.
Here's a diagram of my section:
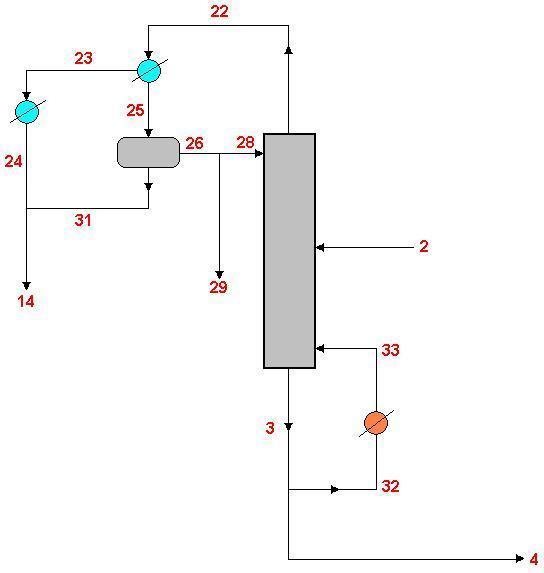
So overhead we should have water and MIBK going to a partial condenser, the liquid phase goes to a gravity separator where MIBK and water separates while the remaining vapour (majority water) goes to a 2nd condenser. MIBK is refluxed to the column from the gravity separator while the water from the separator and 2nd consenser mixes and goes to a raffinate stripper for treatment.
This is a design we agreed on as a group, and was found in a library journal, though I'm slightly confused as to why there should be 2 condensers, why not just condense it all and separate the whole lot?
Anyway my question, I don't know where to start with this design! where do I start?
I'v seen multicomponent distillation examples with light hydrocarbons, propane, butane etc but nothing like the mixture I have. Also it's not exactly a straight forward reflux setup, and have no idea how to model this on Aspen HYSYS.
So basically can anyone help me on where to start? Maybe list the sequence of calculations I should do?
I'v tried a shortcut design on HYSYS, but it doesn't include this reflux setup. Also it only works if I set the liquid fraction of the top product stream to 1, which is confusing, surely 0?
I have done reading through Coulson and Richardson vol 6, but again, I'm not the brightest and still really confused. I think I need to get a proper simulation working, get those K values (vle coefficients) and somehow do a manual calulation (can't rely on HYSYS alone)
If you managed to read this far, I thank you! Not a very clear cut question
I'm currently in my last year of my chemical engineering degree and doing a design project on the manufacture of acrylic acid. I have the job of designing a solvent recovery column, where a mixture of Methylisobutylketone (MIBK) solvent (heavy key) and acrylic acid (light key) are to be separated.
To be honest with you guys I'v not had many good grades throughout my course and it's at the design stages where I suffer as a result, and I'v been stressing over this a lot, time is just passing me by and don't seem to be getting anywhere, so whould really appreciate some help.
So firstly I'll show you my feed composition:
Component-----------------Feed (kg/s) 60*C, 13kPa
Propene-----------------------0
Oxygen------------------------0
Nitrogen-----------------------0.0251
Carbon Dioxide-----------------0.0500
Water-------------------------0.5391
Acrylic Acid--------------------8.5548 (LK)
Acrolein-----------------------7.5365
Acetic Acid--------------------0.2035
MIBK--------------------------29.3277 (HK)
Total---------------------------46.2367
My Assumptions are:
· Key Components: MIBK and Acrylic Acid
· Pseudo-Binary mixture
· 100% recovery of Acrylic & Acetic Acid in bottoms
· 0% solvent & water in bottoms
· From the research I'v done, packing seems to be the way to go for this purpose.
Here's a diagram of my section:
So overhead we should have water and MIBK going to a partial condenser, the liquid phase goes to a gravity separator where MIBK and water separates while the remaining vapour (majority water) goes to a 2nd condenser. MIBK is refluxed to the column from the gravity separator while the water from the separator and 2nd consenser mixes and goes to a raffinate stripper for treatment.
This is a design we agreed on as a group, and was found in a library journal, though I'm slightly confused as to why there should be 2 condensers, why not just condense it all and separate the whole lot?
Anyway my question, I don't know where to start with this design! where do I start?
I'v seen multicomponent distillation examples with light hydrocarbons, propane, butane etc but nothing like the mixture I have. Also it's not exactly a straight forward reflux setup, and have no idea how to model this on Aspen HYSYS.
So basically can anyone help me on where to start? Maybe list the sequence of calculations I should do?
I'v tried a shortcut design on HYSYS, but it doesn't include this reflux setup. Also it only works if I set the liquid fraction of the top product stream to 1, which is confusing, surely 0?
I have done reading through Coulson and Richardson vol 6, but again, I'm not the brightest and still really confused. I think I need to get a proper simulation working, get those K values (vle coefficients) and somehow do a manual calulation (can't rely on HYSYS alone)
If you managed to read this far, I thank you! Not a very clear cut question