- #1
ProblemSets
- 22
- 0
here is how i answered
1=a
2=b,c
3=b
4=a,c
5=a,b,c
what do you guys think? please check it up. Thanks!
1. Which force would cause a compound to have the highest boiling point?
a.) Hydrogen Force b.) Dipole-Dipole Force c.) London Dispersion Force
2. PCl3 would form which force?
a.) Hydrogen Force b.) Dipole-Dipole Force c.) London Dispersion Force
3. Which force is caused by the largest differences in electronegativites?
a.) Hydrogen Force b.) Dipole-Dipole Force c.) London Dispersion Force
4. Which force allows rocks to skip the surface of a lake?
a.) Hydrogen Force b.) Dipole-Dipole Force c.) London Dispersion Force
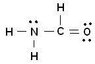
5. Which force would the compound below form?
a.) Hydrogen Force b.) Dipole-Dipole Force c.) London Dispersion Force
1=a
2=b,c
3=b
4=a,c
5=a,b,c
what do you guys think? please check it up. Thanks!
1. Which force would cause a compound to have the highest boiling point?
a.) Hydrogen Force b.) Dipole-Dipole Force c.) London Dispersion Force
2. PCl3 would form which force?
a.) Hydrogen Force b.) Dipole-Dipole Force c.) London Dispersion Force
3. Which force is caused by the largest differences in electronegativites?
a.) Hydrogen Force b.) Dipole-Dipole Force c.) London Dispersion Force
4. Which force allows rocks to skip the surface of a lake?
a.) Hydrogen Force b.) Dipole-Dipole Force c.) London Dispersion Force
5. Which force would the compound below form?
a.) Hydrogen Force b.) Dipole-Dipole Force c.) London Dispersion Force
Attachments
Last edited: