- #1
nonequilibrium
- 1,439
- 2
Hello, I was reading Zeh's "The Physical Basis of the Direction of Time" but I just can't understand him in chapter 1 on something really easy: the definition of the phenomenological entropy + second law. Here is a screenshot I took from the googlebooks edition:
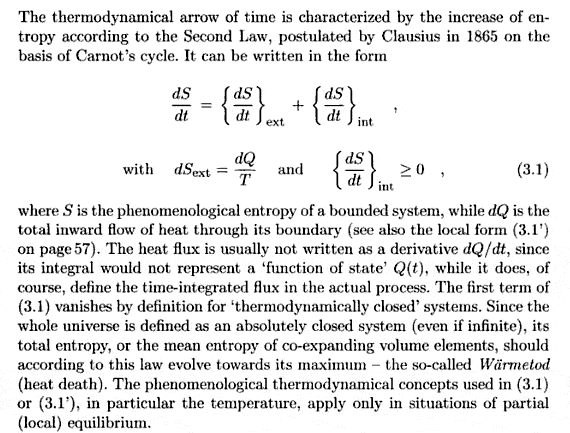
Those two lines of formulas really confuse me, for several reasons. The most obvious one:
He says [tex]\left( \frac{dS}{dt} \right)_{int} \geq 0[/tex]
But I thought the 2nd law clearly stated [tex]\frac{dS}{dt} \right \geq 0[/tex]
After all: a system in connection to a reservoir doesn't have to go up in entropy.
Can anybody clear this up for me?
Those two lines of formulas really confuse me, for several reasons. The most obvious one:
He says [tex]\left( \frac{dS}{dt} \right)_{int} \geq 0[/tex]
But I thought the 2nd law clearly stated [tex]\frac{dS}{dt} \right \geq 0[/tex]
After all: a system in connection to a reservoir doesn't have to go up in entropy.
Can anybody clear this up for me?
