- #1
gfd43tg
Gold Member
- 950
- 50
HelloI have learned about maxwells relations and can derive them. I noticed that we had made an assumption of a closed system.
We just learns about chemical potential and the fundamental relations for an open system. I had a thought experiment that there may exist maxwells relations for an open system that is more general than the ones I previously learned. I tried deriving them in the same way as the closed system equations and attached what I came up with. I was wondering if what I'm trying to do is nonsensical, and if so why is it, or if my math is wrong but I'm on the right track towards something real if I could get some input.
Thanks
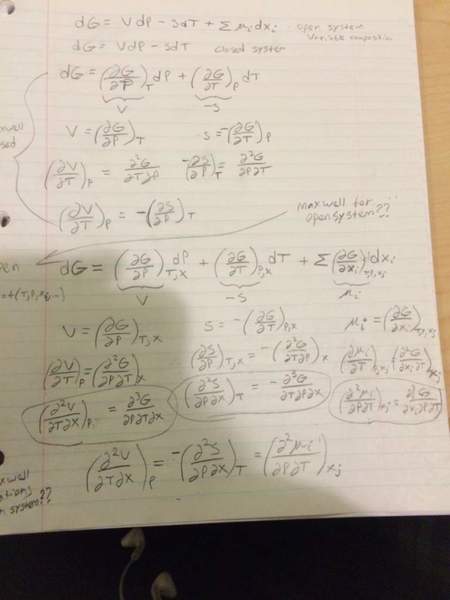
We just learns about chemical potential and the fundamental relations for an open system. I had a thought experiment that there may exist maxwells relations for an open system that is more general than the ones I previously learned. I tried deriving them in the same way as the closed system equations and attached what I came up with. I was wondering if what I'm trying to do is nonsensical, and if so why is it, or if my math is wrong but I'm on the right track towards something real if I could get some input.
Thanks