- #1
aankaa23
- 1
- 0
Homework Statement
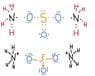
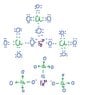
I have to draw Lewis structures and Lewis dot structures of these salts
The Attempt at a Solution
I attached images of how I tried to traw Fe(ClO4)3 and (NH4)2SO3 but I can't figure anything out about Al2(SO4)3
Attachments
Last edited: