- #1
physicsss
- 319
- 0
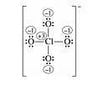
I'm asked to draw 5 possible lewis dot structures for this ion. But I can only think of one. which I 've attached here. I'm supposed to draw them and determine which is the most favorable. I know I can do that by calculating hte formal charges, but first I need to draw these structures. Any suggestions?