- #1
hardyivan007
- 7
- 0
I was taught this in school but my teacher could not explain why the band splits again with decreasing atomic distance. Also, then i wondered how do you explain the fact the it splits again nicely into 4N states for the valence band and 4N states for the conduction band? Could some one help?
Also, how would one redraw the above energy band diagram if the semiconductor was doped to become a n-type or a p-type?
Hope someone could help! Thank You!
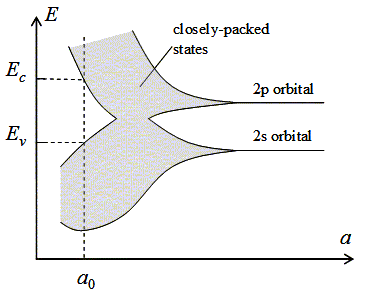
Isolated carbon atoms contain six electrons, which occupy the 1s, 2s and 2p orbital in pairs. The energy of an electron occupying the 2s and 2p orbital is indicated on the figure. The energy of the 1s orbital is not shown. As the lattice constant is reduced, there is an overlap of the electron wavefunctions occupying adjacent atoms. This leads to a splitting of the energy levels consistent with the Pauli exclusion principle. The splitting results in an energy band containing 2N states in the 2s band and 6N states in the 2p band, where N is the number of atoms in the crystal. A further reduction of the lattice constant causes the 2s and 2p energy bands to merge and split again into two bands containing 4N states each. At zero Kelvin, the lower band is completely filled with electrons and labeled as the valence band. The upper band is empty and labeled as the conduction band.
Also, how would one redraw the above energy band diagram if the semiconductor was doped to become a n-type or a p-type?
Hope someone could help! Thank You!
Last edited: