- #1
uchicago2012
- 75
- 0
Homework Statement
I'm trying to figure out the mechanism for a reaction catalyzed by MacMillan Generation 1 and 2 organocatalysts. Not going so well. I don't understand what role the organocatalyst plays in the reaction. I read a paper about MacMillan and I think it was saying that the catalysts worked by forming an imminium ion, but the entire thing was way over my head, so I'm not so sure about that. I drew the reaction without even using the catalyst but my prof said that reaction wouldn't go. I don't really understand how the mechanism works, what the catalyst does to make the reaction 'go,' and how the catalyst makes the reaction enantioselective. Apparently it's a magical substance, I don't know. Can anyone help me with the mechanism? I would feel a great deal better if I knew what these catalysts were running around doing in the reaction.
The Attempt at a Solution
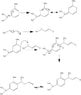
Gen1Genral: General Reaction with Generation One organocatalyst
Gen2General: General reaction with Generation Two organocatalyst
WrongMech: the first mechanism I tried that doesn't have the catalysts and is wrong
NewMech: the newest one I tried that is also probably wrong, seeing as I am stuck (on post under this one)
aldrichchemica: MacMillan paper, in case anyone else can actually understand it (on post under this one)