- #1
simon.23
- 3
- 0
Homework Statement
Toluene is recovered from a mixture of toluene and nitrogen (containing 52 mol% toluene) by cooling the mixture at constant pressure of 1.2 bar to condense out some of the toluene. If the flow rate of the mixture into the condenser is 179 kmol h-1, determine the flowrate of the condensed toluene if the mixture is cooled to a temperature of 25ºC in the condenser. Give your answer in kmol h-1 to an accuracy of one decimal place.
Data: The saturated vapour pressure of toluene in kPa (p*) at TºC is given by:
[tex] log_{10} (p*) = 6.0758-\frac{1342.31}{T +219.19} [/tex]
I have tired this question so many times, and I can't get the right answer, can anyone check my working and guide me as to where I am going wrong?
Thank you.
I know I need to use daltons law to work out the mole fraction first;
[tex] y_{v} = \frac{P^{o}_{v}}{P_{T}} [/tex]
where
[tex] y_{v} [/tex] = mole fraction.
[tex] P^{o}_{v} [/tex] = saturated vapour pressure
[tex] P_{T} [/tex] = total pressure1.2 bar = 120 Kpa
from the temperature given I can work out the sturated vapour pressure of tolunene, after being condensed, and hence the mole fraction.
[tex] log_{10} (p*) = 6.0758-\frac{1342.31}{25 +219.19} [/tex]
[tex] log_{10} (p*) = 0.56 [/tex]
[tex] p = 10^{0.56} = 3.79 kpa [/tex]
so mole fraction of condensed toluene;
[tex] y_{v} = \frac{3.79}{120} \times 100 = 3.16 mol \% [/tex]
so now I have to do a material balance to work out the flowrate of the liquid stream,
I will use a basis of 100 kmol h^{-1} ,ans scale up to 179kmol ^{-1} after the answer.
This is where I am stuck. My material balance just cancels out, I can't seem to make any equations out of it;
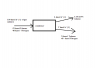
I have attached the material balance diagram, please can someone check it and let me know if it is correct?
Thank you.