- #1
utsav55
- 15
- 0
What will be the % of Enol form of this compound?
99% or 1%? Also give reason.
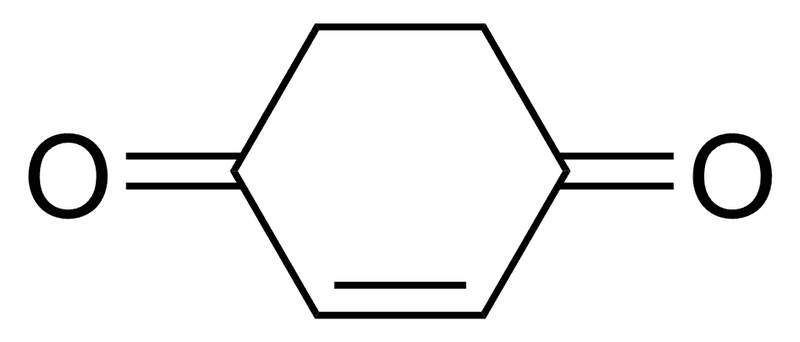
Thanks
99% or 1%? Also give reason.
Thanks
The % of Enol form of a compound is the percentage of the total molecules present in the Enol form. It is calculated by dividing the number of Enol molecules by the total number of molecules and multiplying by 100.
The % of Enol form is typically measured using spectroscopy techniques such as nuclear magnetic resonance (NMR) or infrared (IR) spectroscopy. These methods can differentiate between the Enol and the Keto forms of a compound and determine their relative amounts.
The % of Enol form can be affected by several factors, including the solvent used, temperature, and the presence of other molecules or ions that can stabilize one form over the other. The electronic and steric properties of the compound can also influence the % of Enol form.
The % of Enol form is important because it can affect the reactivity and properties of a compound. For example, the Enol form is generally more reactive than the Keto form and can participate in different reactions. Knowing the % of Enol form can also help in understanding the stability and behavior of a compound in different conditions.
Yes, the % of Enol form can change over time due to various factors such as temperature, pH, and the presence of catalysts. For example, increasing the temperature can favor the formation of the more stable Keto form, which can lead to a decrease in the % of Enol form. Additionally, some compounds can undergo tautomerization, where the Keto and Enol forms can interconvert, further affecting the % of Enol form.