- #1
jincy34
- 20
- 0
Homework Statement
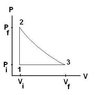
A heat engine using a monoatomic gas follows the cycle shown in Figure. Part 2®3 is adiabat, Vi=101 cm3, Vf=668 cm3, Pi=118 kPa, T1=315 K.
Find Q for process 1 to 2.
Find Ws for process 2 to 3.
Homework Equations
PV=nRT
Q=nCv(delta T)
The Attempt at a Solution
I found moles to be 0.0045. But, I don't know where to go from there. I don't know how to find the tempertaure for step 2 and 3.
please help
the picture is added as an attachment.
Attachments
Last edited: