- #1
Dogsnake
- 1
- 0
Homework Statement
This isn't exactly a homework question, but my lab partner and I came across a phenomena whilst doing an electron spin resonance experiment, and I was wondering if anyone could help explain it.
The sample we found electron spin resonance for is 1,1-diphenyl-2-picrylhydrazil.
A glass phial containing the sample was placed between two Helmholtz coils, so that the B field was uniform, and within the output coil of a radio frequency oscillator. The drop in voltage due to resonance was measured by an oscilloscope in X-Y mode.
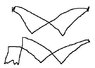
Whilst the radio frequency oscillator was set at 18-35MHz, the waveform shown by the oscillator at resonance was the first shown in the attached file (this was the expected shape). Below a frequency of about 18MHz for the radio frequency oscillator, the waveform looked like the second shown below (excuse my drawing skills, but that's pretty much what it looked like)
The question: Why has this happened?
A qualitative explanation would be fine, as we don't plan on doing experiments on the anomaly itself, we just need to know what's happening. I've spent a while perusing relevant textbooks and can't find a reference to it.
Thank you.