- #1
sci_girl
- 4
- 0
Assume we have a concentration of any specific material (in mg/M). Let's say for example: Ag (Z=47) with concentration 10 mg/M in water (H2O, Z=7.42).
How can I calculate the effective atomic number (Zeff.) for the silver-water mixture if the concentration of silver was 10 mg/M for example?
The only formula I know to calculate Zeff. is the following:
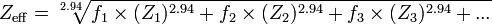
but it uses (fn) the fraction of the total number of electrons associated with each element, not the concentration in mg/M.
Is there any possible way to convert the concentration (in mg/M) into a fraction of total number of electrons for silver then calculate Zeff for the silver-water mixture mentioned above as an example?
Thanks in advance.
source: http://en.wikipedia.org/wiki/Effective_atomic_number (example for H2O provided)
How can I calculate the effective atomic number (Zeff.) for the silver-water mixture if the concentration of silver was 10 mg/M for example?
The only formula I know to calculate Zeff. is the following:
but it uses (fn) the fraction of the total number of electrons associated with each element, not the concentration in mg/M.
Is there any possible way to convert the concentration (in mg/M) into a fraction of total number of electrons for silver then calculate Zeff for the silver-water mixture mentioned above as an example?
Thanks in advance.
source: http://en.wikipedia.org/wiki/Effective_atomic_number (example for H2O provided)