- #1
CrimpJiggler
- 149
- 1
On an exam question recently, I had to perform a retrosynthesis on a molecule and it had this functional group on it:
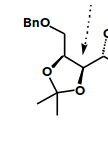
it took me by surprise. I decided to cleave the whole thing off, and replace it with a double bond (cuz I know you can make cis diols from double bonds) then things seemed to fall into place because the double bond let me do a retro diels alder and finish the question, but I couldn't explain how to make that double oxygen thing. I said you could make it with an osmium tetroxide catalysed reaction, but that doesn't explain the t-butyl group. After the exam, my classmates said it was an acetal protected carbonyl group but how can it be an acetal if the oxygens are attached at different carbons?
it took me by surprise. I decided to cleave the whole thing off, and replace it with a double bond (cuz I know you can make cis diols from double bonds) then things seemed to fall into place because the double bond let me do a retro diels alder and finish the question, but I couldn't explain how to make that double oxygen thing. I said you could make it with an osmium tetroxide catalysed reaction, but that doesn't explain the t-butyl group. After the exam, my classmates said it was an acetal protected carbonyl group but how can it be an acetal if the oxygens are attached at different carbons?