- #1
Antuanne
- 23
- 0
I am sort of new to physics and have an interest in particle physics. I was trying to figure out what "magnetic moment" of an electron is and I saw this picture and I am wondering if I am correct about something.
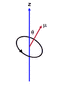
In the diagram I have supplied, does the arrow μ or the circle represent the magnetic moment of a particle? Trying to figure this out. And could this diagram apply to an electron? If not, what's the difference between and electron's and this?
In the diagram I have supplied, does the arrow μ or the circle represent the magnetic moment of a particle? Trying to figure this out. And could this diagram apply to an electron? If not, what's the difference between and electron's and this?
Attachments
Last edited: