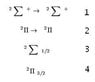- #1
DivGradCurl
- 372
- 0
Hello folks,
I'm reading quite a bit of laser physics material, and I'd like to understand the conventions of the states and transitions. I'm an undergraduate, so my background in quantum mechanics is at the very basic level. I would like to know the meaning of transitions such as the following:
(1)
and
(2)
... and what is the convention behind
(3) and (4)?
Any nice references? Thank you very much!
(the latex in the website is not working right, so I attached an image file with the symbols)
I'm reading quite a bit of laser physics material, and I'd like to understand the conventions of the states and transitions. I'm an undergraduate, so my background in quantum mechanics is at the very basic level. I would like to know the meaning of transitions such as the following:
(1)
and
(2)
... and what is the convention behind
(3) and (4)?
Any nice references? Thank you very much!
(the latex in the website is not working right, so I attached an image file with the symbols)
Attachments
Last edited: