- #1
Hypersphere
- 191
- 8
Hi,
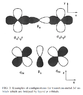
Reading http://rmp.aps.org/abstract/RMP/v70/i4/p1039_1 I've run into a notational question of atomic p orbitals. The authors use symbols like [itex]p_\sigma[/itex] and [itex]p_\pi[/itex]. From their fig. 3 (attached), they do look like [itex]p_x[/itex] and [itex]p_z[/itex] orbitals, respectively, rather than anything close to σ or π bonds.
Is this just a common notational convention that I've somehow missed? And if so, what is the symbol for the third p orbital?
Reading http://rmp.aps.org/abstract/RMP/v70/i4/p1039_1 I've run into a notational question of atomic p orbitals. The authors use symbols like [itex]p_\sigma[/itex] and [itex]p_\pi[/itex]. From their fig. 3 (attached), they do look like [itex]p_x[/itex] and [itex]p_z[/itex] orbitals, respectively, rather than anything close to σ or π bonds.
Is this just a common notational convention that I've somehow missed? And if so, what is the symbol for the third p orbital?