- #1
geoffreythelm
- 11
- 0
Hi, I am having trouble with the following:
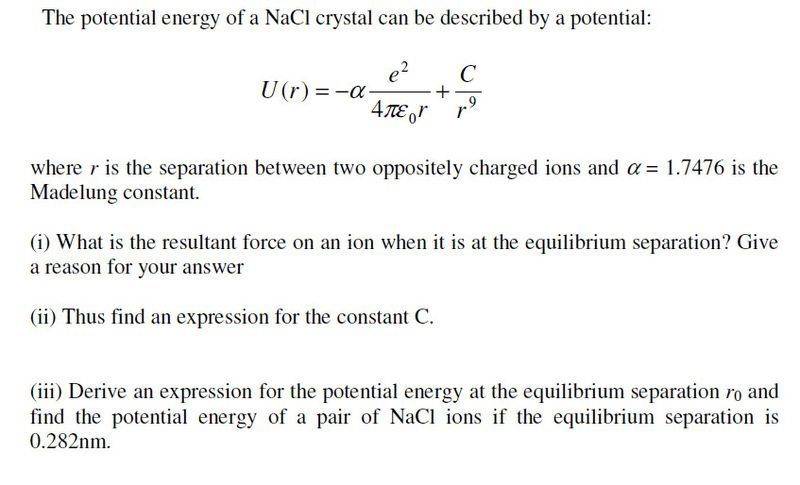
All I have really done is differentiate the function to give an expression for F(r), but I am a bit clueless about the rest. How can there be equilibrium if the two ions are attracted to each other?
Cheers,
GeoffreyThelm
All I have really done is differentiate the function to give an expression for F(r), but I am a bit clueless about the rest. How can there be equilibrium if the two ions are attracted to each other?
Cheers,
GeoffreyThelm
