- #1
mcfaker
- 43
- 0
Hi,
According to the molecular orbital diagram of the (C2)2+ ion you its a stable ion, because it has a bond order of 1 & that means its a stable substance.
Now if you draw the lewis structure you could obtain the geometric arrangement of the electron pairs ( groups) around the central atom. In this case its the following lewis structure:
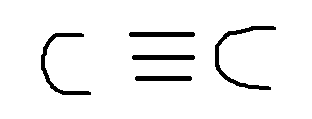
Now here you can find only 1 electron group around the central atom:
Now it if were 2, 3, 4, 5 or 6 you could determine the arrangement of the electron pairs (groups) around the central atom. From this arrangement we could determine the hybridization of the central atom.
But the problem is that there is only 1 electron group, so what is the geometrical arrangement of the electron pairs(groups) around the central atom?
I would like to know if there is hybridization in the (C2)2+ ion, but I can't figure out because of this problem. Could someone please help me?
Thanks in advance!
According to the molecular orbital diagram of the (C2)2+ ion you its a stable ion, because it has a bond order of 1 & that means its a stable substance.
Now if you draw the lewis structure you could obtain the geometric arrangement of the electron pairs ( groups) around the central atom. In this case its the following lewis structure:
Now here you can find only 1 electron group around the central atom:
Now it if were 2, 3, 4, 5 or 6 you could determine the arrangement of the electron pairs (groups) around the central atom. From this arrangement we could determine the hybridization of the central atom.
But the problem is that there is only 1 electron group, so what is the geometrical arrangement of the electron pairs(groups) around the central atom?
I would like to know if there is hybridization in the (C2)2+ ion, but I can't figure out because of this problem. Could someone please help me?
Thanks in advance!