- #1
cvsanchez
- 9
- 0
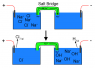
I recently became interested in the chlor-alkali process, specifically the one using a salt bridge, and wondered if I could adapt the setup to synthesize other salts. I have KNO3, but want to remove the nitrate ion and bind it to some other metal, such as copper, and be left with KOH. Of course, this won't work in a regular cell as Cu(OH)2 will just precipitate out. However, I was thinking I could use a salt bridge and set up the cell as follows to produce the Cu(NO3)2:
Cell 1 has the KNO3 solution with a PbO2 anode (+) while cell 2 is a pure water solution with a Cu cathode (-). The cells are connected via a salt bridge (NaCl maybe?) and powered by a 9.6V battery. Would a cell set up like this produce KOH in cell 1 and Cu(NO3)2 in cell 2? If not, why?
Cell 1 has the KNO3 solution with a PbO2 anode (+) while cell 2 is a pure water solution with a Cu cathode (-). The cells are connected via a salt bridge (NaCl maybe?) and powered by a 9.6V battery. Would a cell set up like this produce KOH in cell 1 and Cu(NO3)2 in cell 2? If not, why?