You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
What is Grams: Definition and 60 Discussions
The gram (alternative spelling: gramme; SI unit symbol: g) is a metric system unit of mass.
Originally defined as "the absolute weight of a volume of pure water equal to the cube of the hundredth part of a metre [1 cm3], and at the temperature of melting ice" (later at 4 °C, the temperature of maximum density of water). However, in a reversal of reference and defined units, a gram is now defined as one thousandth of the SI base unit, the kilogram, or 1×10−3 kg, which itself is defined by the International Bureau of Weights and Measures, not in terms of grams, but by taking the fixed numerical value of the Planck constant h to be 6.62607015×10−34 kg⋅m2⋅s−1.
View More On Wikipedia.org
Originally defined as "the absolute weight of a volume of pure water equal to the cube of the hundredth part of a metre [1 cm3], and at the temperature of melting ice" (later at 4 °C, the temperature of maximum density of water). However, in a reversal of reference and defined units, a gram is now defined as one thousandth of the SI base unit, the kilogram, or 1×10−3 kg, which itself is defined by the International Bureau of Weights and Measures, not in terms of grams, but by taking the fixed numerical value of the Planck constant h to be 6.62607015×10−34 kg⋅m2⋅s−1.
View More On Wikipedia.org
-

I How to convert density ratio of grams/ mm^3 into metric tonnes/ m^3
For the dimensions of a right cylinder, I am given three significant digits for the diameter (17.4 mm) and the height (50.3 mm). The formula for the volume of a right cylinder is V = Pi x r^2 x h, which would lead here to Pi x (17.4 mm / 2)^2 x 50.3 mm = 11,960.69354 mm^3 before rounding to 3... -
I
Is percent yield based on grams or moles?
Is percent yield based on grams or moles? Thanks.- i_love_science
- Thread
-
- Tags
- Grams Moles Percent Percent yield Yield
- Replies: 1
- Forum: Chemistry
-
I
Dilution problem when only working with grams
Hi, So I am aware of the C1V1=C2V2 equation but I feel that this is not really applicable here. I attempted to go about it through instinct, so I would take 0.801g/43.883g = 0.0182. And then just multiply 0.0182 with 2.34g, to get 0.0426g. So the answer would be, if I took 2.34 g from the...- Idontknowanything
- Thread
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-

Grams vs. Newtons: Why Do I See Weights Measured in Grams?
Ok so I've asked my physics teacher and a statics teacher as well and I couldn't get a straight answer from either. So here's my questions... Correct me if I'm wrong, but isn't the gram a unit of mass? Not weight? I thought that for mass to become a unit of weight, it has to have to... -
J
MHB Can you calculate the total mass of three parcels at a post office?
At a post office, there are three parcels of different masses. The mass of the first parcel is y grams. The mass of the second parcel is 500 grams greater than that of the first parcel. The third parcel is 210 grams lighter than the first parcel. a) Find the total mass, in grams, of the three...- Johnx1
- Thread
-
- Tags
- Grams Mass Terms Word problem
- Replies: 5
- Forum: General Math
-
Z
Converting grams into Milligrams per 100 grams
Homework Statement What is the ratio of the amount of Vitamin C in 500 grams of orange to the amount of Vitamin C in 500 grams of orange juice? There is a graph associated with this question which is attached. I can't find out the amount of Vitamin C in 500 grams because the data is given...- zak100
- Thread
- Replies: 8
- Forum: Precalculus Mathematics Homework Help
-

A sample of a compound of cesium (Cs) and iodine (I) contain
Homework Statement A sample of a compound of cesium (Cs) and iodine (I) contains 51.16g cesium and 48.84 g iodine, how many grams of cesium can be obtained from 85.5g of this compound? The attempt at a solution I don't really know where to begin. What I have tried was the following: 51.16 g +...- CMATT
- Thread
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
N
How many atoms of oxygen are present in 300 grams of calcium
Homework Statement How many atoms of oxygen are present in 300 grams of calcium carbonate? molar mass of CaCO3 = 100g Homework Equations The Attempt at a Solution I don't know how to proceed. Can anyone please help me out by giving the forulae or steps on how to do this problem.- NeerajKarthi
- Thread
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-

How to calculate time when given distance, mass, and power
Homework Statement I need to calculate the time that it takes for a drag car that is powered by an 8 gram CO₂ cartridge to go across a certain distance. My wooden drag car is 100 grams. It will be traveling a distance of 50 feet. Homework Equations Force= mass x acceleration Power= work /... -

Stoichiometric relationship between liters and grams
My daughter stump me on a question related to liters and grams in stoichiometry. Wondering if someone could help explain. First we know that 1 mole always eqauls 22.4 liters at STP. And 1 mole also always equals the selected element's atomic mass in grams on the periodic table. So... Let's... -
C
Multiplying mass as kg and grams= 2 different results?
Hi, I'm just wondering. If you mulitiply 1kg with 0.5kg you get 0.5kg But if you do the multiplication in grams you get 1000g *500g = 500.000g = 500kg. a dompletely different result. Why is that? How do you avoid this problem?- christian0710
- Thread
- Replies: 3
- Forum: General Math
-

Grams of solute needed to lower vapor pressure of solvent?
Homework Statement Some KCl is dissolved in water at 25°C, where it completely dissociates. The vapor pressure of pure water at 25°C is 28.3 mmHg. Estimate the mass in grams of KCl needed per liter of pure water to reduce the vapor pressure of water at 25°C by 5%. Homework EquationsThe Attempt...- Elvis 123456789
- Thread
- Replies: 36
- Forum: Biology and Chemistry Homework Help
-
K
Finding number of Anions given grams
Homework Statement How many anions are there in 1.50 g of MgBr2 Homework EquationsThe Attempt at a Solution 1.5 g MgBr2 x (1 mol MgBR2/184.3 g MgBr2) x (6.022 x 1023 anions/mol) which gave me answer of 4.91 x 1021 the correct answer is 9.81 x 1021 am i missing a step, or do i have to...- KTiaam
- Thread
-
- Tags
- Grams
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
M
Unit Conversions Grams to Watts
Homework Statement The power available (output) is measured by the velocity at which you are moving and the thrust required to create that movement. NOTE: Conversions for proper units are provided below equation. Power Available: Pout = Tc * v where: Pout = power available (watts) Tc...- MD_Programmer
- Thread
- Replies: 4
- Forum: Engineering and Comp Sci Homework Help
-
P
To increase the temperature of 50 grams of water by 2 Celsius degrees requires
Homework Statement To increase the temperature of 50 grams of water by 2 Celsius degrees requires? Homework Equations The Attempt at a Solution As I understand it takes one caloric to increase 1 gram of water to 1 *C..Correct? since its 50 grams raised by 2*C, I have 50x2= 100 calories?- pmalayavech
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
E
Weight of water from molecular mass to grams per cubic centimeter
I’ve been scratching my head (and working in ms excel) about the following for about 6 hours and would greatly appreciate it if somebody could help me. Basically, I am trying to figure out WHY the density of water is approximately one gram per cubic centimeter (at 1 atm pressure and 4 degrees... -
F
Calculate Mass of Butane & CO2 for 1.5x10^3 kJ Heat
1. What mass of butane in grams is necessary to produce 1.5 x 10^3 kJ of heat? What mass of CO2 is produced? C4H10 + 13/2 O2 --> 4 CO2 + 5 H2O Heat of reaction = -2658 kJ 2. 3. Mass of butane = 33g Mass of CO2 = 176.04g <-- This is wrong and the right answer is 99g CO2...- FLgirl
- Thread
- Replies: 14
- Forum: Biology and Chemistry Homework Help
-
P
How many grams of Zn reacted in this reaction (very easy chemistry question)
Homework Statement The reaction between zinc and hydrochloric acid is carried out as a source of hydrogen gas in the laboratory: Zn(s)+2 HCl(aq)\rightarrow ZnCl_2(aq)+H_2(g) If 325 mL of hydrogen gas is collected over water at 25°C at a total pressure of 748 mm Hg, how many grams of Zn...- PhyIsOhSoHard
- Thread
- Replies: 5
- Forum: Biology and Chemistry Homework Help
-
P
What could 100 grams of tnt destroy?
The title basically summarizes this,and what Modern Day weapon/explosive could this be compared to?- promeus
- Thread
-
- Tags
- Grams
- Replies: 1
- Forum: Other Physics Topics
-
M
Calculating Grams of Helium Needed for Blimp to Rise
Homework Statement Blimps are being considered for use as freight carriers. A scale model rises when filled with helium to a volume of 55.0 dm3. When 1.10 mol He is added to the blimp, the volume is 26.2 dm3. How many more grams of He must be added to make it rise? Assume constant T and P...- Myung
- Thread
- Replies: 5
- Forum: Introductory Physics Homework Help
-
T
Find grams of product using a balanced chemical equation
Homework Statement 2KClO3 → 2KCl + 3O2 How many grams of potassium chloride are produced if 25 g of potassium chlorate decompose Homework Equations The Attempt at a Solution 25g * (1 mole KClO3/122.45 g KClO3) *(74.45g/ 1 mole KCl) =15.2 g KCl is this right??- trivk96
- Thread
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
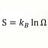
1 gram H / 1 atomic mass unit (grams ) What is wrong with the math here ?
One atomic mass unit = .001660565 x 10-24 grams One gram Hydrogen / .001660565 x10-24 grams = 6.02 x 10 26 ? This should be Avogadros number 6.02 x 1023 what is the mistake here ? -
E
Grams of gasoline needed to raise level of CO in air.
Homework Statement Assume that an incorrectly adjusted lawn mower is operated in a garage such that the combustion reaction in the engion is C8H8. If the dimentions of the garage are 5x3x3 meters. How many grams of gasoline must be burned to raide the levl of CO in the air to 1000ppm by...- eddzzz_2011
- Thread
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
N
Chemistry Converting from Grams to moles
If I have BaCl2 *2H2O how does that come to 8.46507394x10-3 Here is the question: A common laboratory experiment involves the analysis of a mixture of BaCl2 and BaCl2 *2H2O. you are given a white powder that is a mixture of these compounds and are asked to determine the weight percent of...- nieuport27
- Thread
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
L
Find how many grams are required to react with a solution
Homework Statement How many grams of Na2CO3 are required to completely react with 35.6 mL of a .315 M HCL Soliution? Homework Equations Na2CO3 + 2 HCl -> 2 NaCl + CO2 + H20 The Attempt at a Solution 1) Would I find the grams per mol of Na2CO3?- lokobreed
- Thread
-
- Tags
- Grams
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
O
Calculate grams according to equation
i got this equation:2c2h2+5o2->4co2+2h2o how i Calculate how many grams of C2H2 and how many mol of O2 are Necessary to get 0.005 mol Gas CO2? and tell me if i Right: to Calculate the mass ( in gr) of 100 Molecules SO2. I did like this: the mass of SO2 is 64 gr/mol so 100 Molecules... -
C
Mass vs. Period: Should I Convert Grams to Kg?
Homework Statement I did an experiment of mass vs. period and I got the mass in (g)rams and the period in seconds. My question is, do I have to convert the mass from grams to kilograms? Homework Equations N / A The Attempt at a Solution N / A -
C
Density problem to find grams?
Homework Statement A solution is prepared by adding enough water to 185 grams of CsCl to form 1 liter of solution. What is the mass of the solution? The density of the solution is 1.182 g/mL. Homework Equations D= m/v The Attempt at a Solution D= 1.182 g/mL ----> 185 g CsCl /...- Complexity
- Thread
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
4
How many grams CH3COONa do I need to create this buffer?
Problem: I need 750mL of an acetic acid-sodium acetate buffer with pH = 4.3. Solid sodium acetate (CH3COONa) and glacial acetic acid are available. Glacial acetic acid is 99% CH3COOH by mass and has a density of 1.05 g/mL. If the buffer is to be 0.25M in CH3COOH, how many grams of CH3COONa...- 4LeafClover
- Thread
- Replies: 13
- Forum: Biology and Chemistry Homework Help
-
K
Calc Mass KHP Req'd to React w/ 0.100M NaOH - 65 Char
Homework Statement Calculate the mass (in grams) of KHP required to react completely with the NaOH in 35 mL of 0.100M NaOH. KHP = KHC8H4O4(aq) molar mass of "KHP"= 204.2 g/mol Homework Equations This is a double replacement reaction of the acid-base neutralization type. In other words the K...- kremit
- Thread
- Replies: 8
- Forum: Biology and Chemistry Homework Help
-
L
Grams are same when looking for limiting reactant?
Hey, the question was to find the limiting reactant for Lead II Nitrate and Potassium Chromate. I did the work and ended up with 0.084 g for both calculations. (sorry I am not sure how the proper jargon is in chem) So the grams are the same...does that mean that there is not limiting...- legendarium
- Thread
-
- Tags
- Grams
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
C
Grams of H2 react with 28.0 grams of N2
Homework Statement how many grams of H2 are needed to react with 28.0 grams of N2? Homework Equations The Attempt at a Solution I believe the answer to be 14 grams or 6 grams. I keep going back and forth between the 2. Any help would be greatly appreciated, thanks!- Chuck Norris
- Thread
-
- Tags
- Grams
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
K
Convert ml to grams for a Heat Capacity and Specific Heat problem, is that possible?
Homework Statement Is it possible to convert ml to grams for a Heat Capacity and Specific Heat type of problem? The problems that I have done so far did not use ml at all. I am stuck with the following problem: A piece of stainless steel (specific heat = 0.50 J g-1 oC-1 ) is taken from an...- Knight226
- Thread
- Replies: 3
- Forum: Biology and Chemistry Homework Help
-
I
A piece of iron weighing 85.65 grams was burned in air.
Homework Statement A piece of iron weighing 85.65 grams was burned in air. The mass of the iron oxide produced was 118.37g. 1. Use the law of conservation of mass to calculuate the mass of oxygen that reacted with iron. 2. use the molar mass of oxygen to calculate the number of moles... -
P
How many grams of cold water necessary to lower temperature in container
Homework Statement How many grams of 20 degree C water do I need to add to 100 grams of 80 degree C of water to get the final temperature down to 50 degrees C? Homework Equations I'm sure it's a pretty simple equation, but I don't know it. (I'm studying a lot of different things at once...- Paulo Serrano
- Thread
-
- Tags
- Cold Container Grams Temperature Water
- Replies: 3
- Forum: Introductory Physics Homework Help
-
H
How many grams of Fe needed?
1. Homework Statement [/b] Given: C2CL4 + Fe \rightarrow C2H4 + CL- + Fe2+ A reservoir contains 5million gallons of water which is contaminated with 4.2mg/L of Perchloroethene(C2CL4). You want too reduce it to harmless ethene(C2H4) by reacting it with Fe. because of this only about 20g...- hils0005
- Thread
-
- Tags
- Grams
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
T
Converting Moles to Grams and Vice Versa: Potassium Sulfide and Barium Nitride
Homework Statement How many grams are there in 6.80 moles of potassium sulfide? How many moles are there in 200g of barium nitride? Homework Equations I just want to see if i got the right answer The Attempt at a Solution for part A. i got 759g by multiplying 6.80x110.24(molar mass...- tommyjohn
- Thread
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
D
Calculating Mass of Electrons in GRAMS
Electrons in GRAMS :( Homework Statement Part1: A sample of electrons is found to have a total charge of -7.68 x 10^8 C. How many electrons are in this sample ? =================================== Part 2 says: What is the mass of the above sample of electrons in grams ...- deteam
- Thread
- Replies: 9
- Forum: Biology and Chemistry Homework Help
-
R
Calculating Atomic Mass: How Many Grams in 1 amu?
Homework Statement I am reviewing some very general chemistry and for some reason I cannot come up with a method of approach for this problem? I know that there are 1 amu/atom and that there are 1g/mol and that there are 6.022*10^24 atoms/mol. How do I put it all together?- Roger Wilco
- Thread
-
- Tags
- Grams
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
M
Chemistry How many grams of H2O are produced when 2.5 moles of O2 are used?
Hi I need help with a Stoichiometry problem. I am complete lost. I don't know where to start, or how to to solve. 2H2 + O2 -2H2O. I know the formula balances and that is it. The questions is How many grams of H2O are produced when 2.5 moles of O2 are used? Thanks!- magaly79
- Thread
- Replies: 3
- Forum: Biology and Chemistry Homework Help
-
T
How much power will 10 grams of plutonium produce?
How much power will 10 grams of plutonium produce in megatons if 100% of the 10 grams is converted into energy?- The000Agent
- Thread
- Replies: 5
- Forum: High Energy, Nuclear, Particle Physics
-
S
Chemistry Atoms, Moles, Grams Conversion
Homework Statement A microscopic scientist is assembling sugar molecules (C6H12O6) from C, H, and O atoms. If he has 6.022x10^23 atoms of each type, how many grams of sugar molecules can he assemble? Which type of atom does he run out of first? Homework Equations 1 mol = 6.02x10^23 atoms 1...- Spartan Erik
- Thread
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
M
Calculate the mass in grams of each element in the body of a 57kg person
Homework Statement The natural abundaces of elements in the human body, expressed as a percent by mass, are oxygen (o) 65%; carbon (C), 18%; hydrogen (H), 10%; nitrogen (N), 3.0%; calcium (Ca), 1.6%; phosphorous(P), 1.2%; all other elements 1.2% Calculate the mass in gram of each element in...- mightyhealthy
- Thread
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
C
Calculating the Number of Grams of Ethanedioate Ion in 1 dm3
Hi, i wonder how could i calculate the number of grams of ethanedioate ion, C2O42- in one dm3 Given: FA1 was made by dissolving 6 g of metal ethanedioate, MC2O4 is dissolved in dilute sulpuhric acid and making up to 1 dm3 with pure water. FA2 contains 2.38g of manganate (VII) ion per...- crays
- Thread
- Replies: 3
- Forum: Biology and Chemistry Homework Help
-
B
Burning Calories, Burning Wood, Grams to Cals?
Alright so I apologize if this questions seems less "physics" based, but I promise there is a reason I'm posting it here- if you can thoroughly explain this, I'd much appreciate it! FIRST PREMISE: I was recently reading on the forum about the question of burning a log of wood (mass) and...- Besowonderous
- Thread
- Replies: 21
- Forum: Other Physics Topics
-
M
Grams and volume of steam needed to raise the temperature of expresso
[SOLVED] Grams and volume of steam needed to raise the temperature of expresso Homework Statement The temperature of espresso coffee (mostly water) can be increased by blowing 100°C steam into it. How much steam (in grams) is needed to heat up a 13 cm3 cup of espresso from 46°C to 82°C...- merbear
- Thread
-
- Tags
- Grams Steam Temperature Volume
- Replies: 2
- Forum: Introductory Physics Homework Help
-
J
Solving for Grams of Citric Acid in Juice Solution
[SOLVED] Titration Lab Homework Statement How many grams of pure citric acid in the juice solution when 4.00 g of the juice crystals are dissolved in 100.0 mL solution? The juice solution is titrated using a given solution of sodium hydroxide. The NaOH is titrated into 10 mL of the juice...- jumbogala
- Thread
-
- Tags
- Acid citric Citric acid Grams
- Replies: 12
- Forum: Biology and Chemistry Homework Help
-
B
8. When 15 grams of H2O decomposes how many grams of O2 are formed?
[SOLVED] 8. When 15 grams of H2O decomposes how many grams of O2 are formed? Here's the question: When 15 grams of H2O decomposes how many grams of O2 are formed? I have no idea how to even start that problem...I guess I missed that part in Chem class. *sighs*- BittersweetLove
- Thread
-
- Tags
- Grams
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
H
Calculating grams of Precipitate
Homework Statement If 70.0 mL of 0.150 M CaCl2 is added to 15.0 mL of 0.100 M AgNO3, what is the mass in grams of AgCl precipitate? Homework Equations Limiting reagants. The Attempt at a Solution So I tried to find the limiting reagent, but that's where I'm stuck. I'm using examples...- hviz
- Thread
-
- Tags
- Grams
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
N
Solving for n: How Many Grams of Hydrogen in 6.25L @ 1.95ATM & 243K?
If there are 6.25 L of Hydrogen gas at a pressure of 1.95 ATM and a temperature of 243 K, how many grams of hydrogen are there? I know that the equation for the ideal gas law is: pV = nRT p = pressure V = volume n = number of moles R = the gas constant, 0.0821 L atm mol-1 K-1 T =...- n108
- Thread
- Replies: 2
- Forum: Biology and Chemistry Homework Help