You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
What is Periodic table: Definition and 93 Discussions
The periodic table, also known as the periodic table of elements, is a tabular display of the chemical elements, which are arranged by atomic number, electron configuration, and recurring chemical properties. The structure of the table shows periodic trends. The seven rows of the table, called periods, generally have metals on the left and nonmetals on the right. The columns, called groups, contain elements with similar chemical behaviours. Six groups have accepted names as well as assigned numbers: for example, group 17 elements are the halogens; and group 18 are the noble gases. Also displayed are four simple rectangular areas or blocks associated with the filling of different atomic orbitals.
The elements from atomic numbers 1 (hydrogen) to 118 (oganesson) have all been discovered or synthesized, completing seven full rows of the periodic table. The first 94 elements, hydrogen to plutonium, all occur naturally, though some are found only in trace amounts and a few were discovered in nature only after having first been synthesized. Elements 95 to 118 have only been synthesized in laboratories, nuclear reactors, or nuclear explosions. The synthesis of elements having higher atomic numbers is currently being pursued: these elements would begin an eighth row, and theoretical work has been done to suggest possible candidates for this extension. Numerous synthetic radioisotopes of naturally occurring elements have also been produced in laboratories.
The organization of the periodic table can be used to derive relationships between the various element properties, and also to predict chemical properties and behaviours of undiscovered or newly synthesized elements. Russian chemist Dmitri Mendeleev published the first recognizable periodic table in 1869, developed mainly to illustrate periodic trends of the then-known elements. He also predicted some properties of unidentified elements that were expected to fill gaps within the table. Most of his forecasts soon proved to be correct, culminating with the discovery of gallium and germanium in 1875 and 1886 respectively, which corroborated his predictions. Mendeleev's idea has been slowly expanded and refined with the discovery or synthesis of further new elements and the development of new theoretical models to explain chemical behaviour. The modern periodic table now provides a useful framework for analyzing chemical reactions, and continues to be widely used in chemistry, nuclear physics and other sciences. Some discussion remains ongoing regarding the placement and categorisation of specific elements, the future extension and limits of the table, and whether there is an optimal form of the table.
View More On Wikipedia.org
The elements from atomic numbers 1 (hydrogen) to 118 (oganesson) have all been discovered or synthesized, completing seven full rows of the periodic table. The first 94 elements, hydrogen to plutonium, all occur naturally, though some are found only in trace amounts and a few were discovered in nature only after having first been synthesized. Elements 95 to 118 have only been synthesized in laboratories, nuclear reactors, or nuclear explosions. The synthesis of elements having higher atomic numbers is currently being pursued: these elements would begin an eighth row, and theoretical work has been done to suggest possible candidates for this extension. Numerous synthetic radioisotopes of naturally occurring elements have also been produced in laboratories.
The organization of the periodic table can be used to derive relationships between the various element properties, and also to predict chemical properties and behaviours of undiscovered or newly synthesized elements. Russian chemist Dmitri Mendeleev published the first recognizable periodic table in 1869, developed mainly to illustrate periodic trends of the then-known elements. He also predicted some properties of unidentified elements that were expected to fill gaps within the table. Most of his forecasts soon proved to be correct, culminating with the discovery of gallium and germanium in 1875 and 1886 respectively, which corroborated his predictions. Mendeleev's idea has been slowly expanded and refined with the discovery or synthesis of further new elements and the development of new theoretical models to explain chemical behaviour. The modern periodic table now provides a useful framework for analyzing chemical reactions, and continues to be widely used in chemistry, nuclear physics and other sciences. Some discussion remains ongoing regarding the placement and categorisation of specific elements, the future extension and limits of the table, and whether there is an optimal form of the table.
View More On Wikipedia.org
-

Reactivity in Different Groups
ik this is basic knowledge, that all groups go up in reactivity the further down you go in the group, except for group 7, where this is reversed. however i don't understand why, because in group 7, the electron shielding still increases the further down the group you go, like with all the other... -
J
I Some questions about this vintage periodic table
I was recently in an art supply store and saw a print of a vintage periodic table that I thought had real aesthetic value. I bought it and like any enthusiast I hung it in my bathroom. For the past month I have been studying it as I brush my teeth and feel comfortable with 80% of the...- jmatt
- Thread
-
- Tags
- Nitrogen Periodic table
- Replies: 10
- Forum: Quantum Physics
-

LaTeX Most awesome periodic table of the elements in Latex?
I would like to print a nice PTOTE, written in Latex. So that I could make small modifications before printing it. I have checked on the Internet, but surprisingly couldn't find any really nice one. Ideally, it should contain information like the fusion point, possibly crystallographic...- fluidistic
- Thread
- Replies: 2
- Forum: MATLAB, Maple, Mathematica, LaTeX
-

The shielding effect and effective nuclear charge
Whats shielding effect and effective nuclear charge? -

Energy of valence electrons from period of the periodic table
I am currently studying Electrical Engineering and I have this question: An energy band is formed by the overlapping of atomic orbitals of atoms coming close to each other.I suspect that if the energy of the atomic orbital of the valence electrons of a chemical element is less than the energy of...- Helena Wells
- Thread
- Replies: 2
- Forum: Atomic and Condensed Matter
-
S
B Does Dark Matter Have a Periodic Table?
Hello All There is much discussion on the existence of Dark Matter. Should we think of Dark Matter as having macro structure, ie comprising elementary particles, leading to atoms and a Dark Periodic Table? best regards ... Stef- saddlestone-man
- Thread
- Replies: 12
- Forum: Cosmology
-
F
Radioactivity and the Periodic Table
Hello, The periodic table organizes all known chemical elements (total of 118) based on their atomic number and properties. My understanding is that the first 92 elements are commonly found in nature while the other 26 are either highly radioactive and/or artificially made. Radioactive elements... -

B Is there a Relativistic Quantum Chemistry Table?
Wiki said "Arnold Sommerfeld calculated that, for a 1s orbital electron of a hydrogen atom with an orbiting radius of 0.0529 nm, α ≈ 1/137. That is to say, the fine-structure constant shows the electron traveling at nearly 1/137 the speed of light.[9] One can extend this to a larger element with...- shintashi
- Thread
- Replies: 1
- Forum: Atomic and Condensed Matter
-
M
Ray Kurzweil and What elements could exotic baryons create?
I was reading the Exotic baryons page on Wikipedia and a cited source said that Ray Kurzweil, renowned Futurist said that at the end of the 21st century, we could use femtotechnology to create new chemical elements from exotic baryons that could create a new periodic table of elements. Here is...- Maximum7
- Thread
-
- Tags
- Baryons Elements Periodic table Ray
- Replies: 2
- Forum: Science Fiction and Fantasy Media
-
B
I Why does the arrangement of the Periodic Table make no sense?
We're told that the electron shells give the relative reactivity/affinity to other atoms by their incompleteness, completeness or over-completeness, and arranged in the periodic table accordingly as columns. The shells are are concentrically arranged around the nucleus from smaller capacity to...- Bonkus Klunkit
- Thread
-
- Tags
- Periodic Periodic table Table
- Replies: 2
- Forum: Classical Physics
-

Computer code that handles the periodic table of elements
Hi guys When I ran the Chemical Equilibrium Code from NASA (grc.nasa.gov) to predict the products of a chemical reaction and their concentrations, it says it does not handle certain elements in the database from the periodic table. Is there a computer code that predicts the products of a...- Adrian Tudini
- Thread
- Replies: 5
- Forum: Chemistry
-

Transform the periodic table of chemical elements.
Summary: Transform the periodic table of chemical elements (periodic table) into a universal way of storing and transmitting information using spectral analysis. I propose a concept in which the basis for working with information (conservation, transmission in networks) is to use spectral...- Max Kovalevich
- Thread
- Replies: 15
- Forum: Electrical Engineering
-
R
How did 19th century scientists measure atomic and molecular weight?
Found myself wondering about this recently, though I can't recall the context. When Mendeleev proposed the periodic table of elements, I believe that it was known that atomic weights of the known elements were multiples of hydrogen's atomic weight. Presumably also with substances like oxygen... -

How Has the Periodic Table Changed Over its 150 Year History?
The Periodic Table is 150 years old sometime this year (I could not find its exact birthday). Good job Mendeleev! Here is a Science magazine news info graphic on how it has changed over time (before and after Mendeleev). The graphic came out a while ago, but was not working then. Now it does.- BillTre
- Thread
-
- Tags
- Periodic Periodic table Table
- Replies: 3
- Forum: Chemistry
-
I
MHB Help with Periodic Table Question, don't know what I'm doing wrong.
Help with this- ilovewatermelon
- Thread
-
- Tags
- Periodic Periodic table Table
- Replies: 1
- Forum: General Math
-
I
MHB Help with periodic Table question, express the area in square meters
- ilovewatermelon
- Thread
- Replies: 3
- Forum: General Math
-

Why was the F block of the periodic table created?
Basically, the F block is a series in the periodic table that consist of elements that are artificially synthesized. My question is, why were these elements synthesized? What was the need of synthesizing such elements? -

Oddly specific number of elements
There are 118 elements known to man, and some scientists like Feynman think that element 137 might be the end of the Periodic Table. Isn't that oddly specific? To me, it feels like it is completely random and of no significance. What is going on here? Is there a constant that relates to this... -
R
Courses What other chemistry courses use the periodic table?
So far, I have taken General Chemistry I and II, and Organic Chemistry I. Out of these classes, only General Chemistry I seems to make use of the periodic table, but it is mostly just going through the basics of the periodc table. Not so much in Gen Chem II or Orgo I. I mean they give it to you...- Ric-Veda
- Thread
- Replies: 4
- Forum: STEM Academic Advising
-
I Which are semiconductors in the periodic table?
Can combination elements in group I and group VII be a semiconductor? My thinking is that they form the octet rule just like group II and group VI elements.- bluejay27
- Thread
- Replies: 1
- Forum: Atomic and Condensed Matter
-

Periodic table - certain no. of atoms in the 1st 4 rows
Homework Statement Explain why the first four rows of periodic table have 2, 8, 8 and 18 atoms respectively Homework Equations I have a feeling this has something to do with the central field approximation OR the s, p, d, f orbitals and how many electrons can go in each OR something else The...- Ags Ivana
- Thread
-
- Tags
- Atoms Periodic Periodic table Table
- Replies: 1
- Forum: Introductory Physics Homework Help
-

Choose the element in each of the sets you would expect to have the highest IE2
Homework Statement Choose the element in each of the sets you would expect to have the highest IE2. a. K b. Be c. Mg d. Ca e. Al Homework Equations The correct answer is K The Attempt at a Solution I do not understand why it is K ...I kind of guessed by using my Ionization Energy diagram...- CMATT
- Thread
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
F
Periodic Table Element Names: Latin vs. Germanic
Hi. Why do some countries use different names for the elements instead of their original names as indicated by their symbol? Like Na(natriu) is called sodium, Au(aur) is gold, Fe(fier) is iron, Cu(cupru) is copper, Ag(argint) is silver, Pb(plumb) is lead. I'm from a Latin/francophone country and...- Fizica7
- Thread
-
- Tags
- Periodic Periodic table Table
- Replies: 12
- Forum: General Discussion
-

Comparing Reactivity: F, Cl, and Br in the Periodic Table
Amoung three elements F,CL,Br which is more reactive? I guessed it to be bromine because it has three shells and hence when we go down the group on the basis of their shells bromine will easily lose electrons. But the book says it is fluorine since it can easily gain electrons.And how can that...- Anithadhruvbud
- Thread
-
- Tags
- Periodic Periodic table Table
- Replies: 3
- Forum: Biology and Chemistry Homework Help
-

Periodic Table and oxidising agent
Homework Statement (i) An element that has a molecule which contains exactly four atoms. (ii) An element that reacts with water to give a solution that can behave as an oxidising agent. Homework EquationsThe Attempt at a Solution (i) Shouldn't it be boron? Boron forms BF3 which has four...- Priyadarshini
- Thread
-
- Tags
- Periodic Periodic table Table
- Replies: 14
- Forum: Biology and Chemistry Homework Help
-

7th period of the periodic table now complete
http://www.iupac.org/news/news-detail/article/discovery-and-assignment-of-elements-with-atomic-numbers-113-115-117-and-118.html -

Explaining the Proton-to-Electron Ratio in the Periodic Table
Hey guys we say that when in periodic table we go from left to right atomic size decreases because of increase in nuclear charge in the same shell but my question is that the electron to proton ratio is 1:1 then how nuclear charge is increased. Some say nuclear charge is concentrated but my...- Karan Punjabi
- Thread
-
- Tags
- Periodic Periodic table Ratio Table
- Replies: 5
- Forum: Other Physics Topics
-

Calculating Energy from Mass Using E=mc²
Homework Statement I have the question with a diagram posted in the thumbnail to make things easier. Homework Equations E = mc² The Attempt at a Solution The homework only had a single example for a question like this so I am not 100% sure how to get the answer. So far I've added the 2...- ElegantSir
- Thread
- Replies: 7
- Forum: Introductory Physics Homework Help
-
B
"Periodic table" of combined elements
The periodic table is globally known! But, it relates EACH element separately! Exist some table, some lists, some spreadsheet, etc, that relates the combinations between the elements and give us the information about each combination? PS: for "combined elements" I want to say simples substance...- Bruno Tolentino
- Thread
-
- Tags
- Elements Periodic table Table
- Replies: 5
- Forum: Chemistry
-
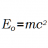
Proposed Rearrangement of Periodic Table
I'm sorry for being so vague here, but perhaps my post will ring a bell for someone who can fill in the details. A few weeks ago in a waiting room I was reading about a proposed rearrangement of the Periodic Table based on measurements of the ionization potentials (I think I got that right) of...- Mister T
- Thread
-
- Tags
- Periodic Periodic table Table
- Replies: 8
- Forum: Atomic and Condensed Matter
-

Insights It's Elemental. The Periodic Table quiz - Comments
Greg Bernhardt submitted a new PF Insights post It's Elemental! The Periodic Table Quiz Continue reading the Original PF Insights Post.- Greg Bernhardt
- Thread
-
- Tags
- Periodic Periodic table Quiz Table
- Replies: 46
- Forum: Chemistry
-
R
Is 5s2 5p4 in Group 17 and Period 6 on a Different Periodic Table?
Homework Statement In the long form of periodic table, the valence shell electronic configuration of 5s2 5p4 corresponds to the element present in A. Group 16 and period 5 B. Group 17 and period 6 C. Group 17 and period 5 D. Group 16 and period 6 Homework Equations NA The Attempt at a...- Raghav Gupta
- Thread
-
- Tags
- Periodic Periodic table Table
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
S
Question about the periodic table arrangement
Hi, I'm new here. I'm actually studying the periodic table and while collectting the data on excel i noticed something. I wouldn't see it with the usual display of a periodic table but when you put it all in a sheet, ligns after ligns it become obvious to me. Argon Ar (noble gaz) is followed... -

Which mass number is shown on the periodic table?
There are many different mass numbers for a given element known as isotopes. Are elements on the periodic table isotopes of their element? Why not use the average of all the mass numbers for an element?- Jewish_Vulcan
- Thread
-
- Tags
- Mass Periodic Periodic table Table
- Replies: 11
- Forum: Chemistry
-
R
What are the most reactive elements in the periodic table?
What are the most reactive elements in the periodic table? -
C
Where should hydrogen be placed in the periodic table?
Hi all, I was always of the belief that hydrogen did not belong to any group in the periodic table. After discussions, some say that a group 1 or 7 place might be more suitable. Any opinions would be welcomed hopefully from a physics point-of-view on this topic.Thanks in advance -
Q
Theory or Reality - Acid/base trends on the periodic table
Homework Statement Number 12. Ignore the scribbling and the circled answers. http://i.minus.com/i17OAHo9PELaW.jpg Homework Equations The periodic table trend of acid/base behavior says that oxides of elements on the right of the periodic table will behave as acids in water. It...- Qube
- Thread
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-

Periodic Table: Transition Metals
1. Regarding: Transition metals of the Periodic Table 2. Here's my question: the D-Block transition metals will always lose e- (& never gain e-'s) to fully fill (or half-fill) their d-subshells, right? 3. Given what I learned about stable, fully-filled and half-filled subshells...- Astronomer1
- Thread
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
M
Atomic weight and noble gases in Mendelev's periodic table
Why did Mendelev order the elements according to their atomic masses rather than their atomic number? Why did Mendelev not include noble gases in his periodic table?- mahrap
- Thread
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
A
Periodic Table/ Elements toxic to one another?
So Arsenic is poisonous to Oxygen based life forms (Humans). What would be poisonous to a Sulfur based life form (if they exsisted)?- artsyashley88
- Thread
- Replies: 2
- Forum: Biology and Medical
-
K
Kitaev's Periodic Table (of Topological Insulators & SCs)
Hi PF, I'm trying to come to grips with the work of Alexei Kitaev on applying notions from (topological) K-theory to the task of classifying phases of topological insulators and superconductors (paper here: http://arxiv.org/pdf/0901.2686v2.pdf). Despite having plenty of citations, I've yet to...- Kirjava
- Thread
- Replies: 20
- Forum: Atomic and Condensed Matter
-

Why do modern periodic tables show eighteen groups instead of eight?
Hello.It seems that older periodic tables showed just eight groups but most modern periodic tables now show eighteen.Are there any reasons why eight used to be preferred and why eighteen is now chosen?I'm guessing that the change over is due to...well I don't know.Thanks for any answers.- Dadface
- Thread
-
- Tags
- Periodic Periodic table Table
- Replies: 2
- Forum: Chemistry
-
H
Should the periodic table be revised to this?
Any opinions would be appreciated.- Harmony360
- Thread
-
- Tags
- Periodic Periodic table Table
- Replies: 1
- Forum: Chemistry
-
A
Discuss the evidence from the periodic table
Homework Statement Discuss the evidence from the periodic table of the need for a fourth quantum number. How would the properties of He differ if there were only three quantum numbers, n, l, and m? Homework Equations The Attempt at a Solution The Pauli Exclusion Principle...- awat
- Thread
- Replies: 15
- Forum: Advanced Physics Homework Help
-
T
What are the criteria for classifying elements as metalloids and halogens?
I'm currently studying grade 11 chemistry... And the periodic table of elements slightly confuses me. I am having trouble understanding which are metalloids, and halogens. In the handout that my teacher gave me, it listed the following as metalloids: B, Si, Ge, As, Sb, Te, Po, and At. It also...- thesparrow
- Thread
- Replies: 1
- Forum: Chemistry
-
L
How to memorize the periodic table?
So for my inorganic class we need to know the periodic table by heart, as we will not be getting one on our tests. While memorizing groups and periods in order doesn't hold that much practical sense (it would be much better to be able to just recall any element by it's number along with it's...- LogicX
- Thread
-
- Tags
- Periodic Periodic table Table
- Replies: 4
- Forum: Chemistry
-
S
Why in periodic table normally we have elements with FCC and HCP structure?
what is basic principle followed by atoms to arrange in basic structure?- swapneel5
- Thread
- Replies: 1
- Forum: Atomic and Condensed Matter
-
S
Periodic table of shapes to give a new dimension to maths
Mathematicians are creating their own version of the periodic table that will provide a vast directory of all the possible shapes in the universe across three, four and five dimensions, linking shapes together in the same way as the periodic table links groups of chemical elements. The...- Synetos
- Thread
- Replies: 1
- Forum: Differential Geometry
-
F
Exploring Lanthanide and Actinide Series Exclusion from Periodic Table
why aren't the lanthanide and actinide series included in the periodic table? is there some non periodicity in there elements? -
F
The missing elements in the Periodic Table?
How did Dimitri Mendeleev know that some of the elements were missing/not discovered? How did he know that the missing elements existed? Did he find some sort of pattern when he created the Periodic Table?- FantasyQueen
- Thread
- Replies: 4
- Forum: Chemistry