You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
What is Compressibility: Definition and 59 Discussions
In thermodynamics and fluid mechanics, the compressibility (also known as the coefficient of compressibility or, if the temperature is held constant, the isothermal compressibility) is a measure of the relative volume change of a fluid or solid as a response to a pressure (or mean stress) change. In its simple form, the compressibility
κ
{\displaystyle \kappa }
(denoted β in some fields) may be expressed as
κ
=
−
1
V
∂
V
∂
p
{\displaystyle \kappa =-{\frac {1}{V}}{\frac {\partial V}{\partial p}}}
,where V is volume and p is pressure. The choice to define compressibility as the negative of the fraction makes compressibility positive in the (usual) case that an increase in pressure induces a reduction in volume. The reciprocal of compressibility at fixed temperature is called the isothermal bulk modulus.
View More On Wikipedia.org
κ
{\displaystyle \kappa }
(denoted β in some fields) may be expressed as
κ
=
−
1
V
∂
V
∂
p
{\displaystyle \kappa =-{\frac {1}{V}}{\frac {\partial V}{\partial p}}}
,where V is volume and p is pressure. The choice to define compressibility as the negative of the fraction makes compressibility positive in the (usual) case that an increase in pressure induces a reduction in volume. The reciprocal of compressibility at fixed temperature is called the isothermal bulk modulus.
View More On Wikipedia.org
-

Engineering Exploring the Confusing Impact of Compressibility on CL Measurements
This situation is really confusing me. First of all, does accounting compressibility affect the measurements for CL? If yes, how does CL change as a function of the increasing mach number? Back to the problem, above is the "in-flight" lift curve for a plane traveling at high speeds, so the CLs...- greg_rack
- Thread
- Replies: 1
- Forum: Engineering and Comp Sci Homework Help
-

B NIST database and compressibility factor Z
There's a huge volume of data on NIST database: https://webbook.nist.gov/cgi/fluid.cgi?T=293.15&PLow=10&PHigh=1000&PInc=10&Applet=on&Digits=5&ID=C7727379&Action=Load&Type=IsoTherm&TUnit=K&PUnit=bar&DUnit=mol%2Fl&HUnit=kJ%2Fmol&WUnit=m%2Fs&VisUnit=uPa*s&STUnit=N%2Fm&RefState=DEF I'm interested...- FranzSC
- Thread
- Replies: 1
- Forum: Classical Physics
-
R
A substance has an isothermal compressibility kappa = (aT^3)/(P^2)...
Starting from v(P,T), dv=(dv/dp)_T dp + (dv/dT)_P dt i implemented conditions when T and P are constant and ended up with ln V = aT^3/P + constant and ln V = bT^3 /3P + constant If i assume that the constant is 0, i can say that a/b = 1/3 but how do i justify this assumption?- romanski007
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-
F
B Exploring Bulk Modulus: Examining Caesium and Beyond
Today, while studying about bulk Modulus, I encountered a doubt. Please consider this thought experiment. I'm considering Caesium as an example as it seems to have a quite low Bulk Modulus (comparatively) of 1.6 GPa. Let's say I apply a pressure of X GPa. Volume change ratio can be given by...- Fitz Watson
- Thread
- Replies: 5
- Forum: Other Physics Topics
-

Accoustic waves notions (propagation in a tube)
Please help me with this problem I am facing, I am lacking notions of acoustics and I would be very grateful if someone could clarify them: A tube has a revolution symmetry arounf the ##x## axis and has a section dependent of the value of the abscissa (x), so the profile ##S(x)## is known. The...- Cathr
- Thread
- Replies: 3
- Forum: Introductory Physics Homework Help
-

How to find the thermal compressibility of a gas
Homework Statement : [/B] A gas obeying the equation of state PV=RT undergoes a hypothetical reversible process PV^\frac{5}{3} e^\frac{-PV}{E_0} = c_1 Can we prove that the thermal compressibility of the gas undergoing this process tends to a constant value at very high temperature? Here, E_0...- PKM
- Thread
- Replies: 3
- Forum: Introductory Physics Homework Help
-
S
Pressure trace of a tank fed by a compressor
G'Day All, This is my first post so please let me know if I have completed this form incorrectly, or missed a point of etiquette etc... 1. Homework Statement The problem is to determine the pressurisation rate of a tank being filled by a pipe connected to a compressor. Assumptions: Pipe...- Sweepy
- Thread
- Replies: 1
- Forum: Engineering and Comp Sci Homework Help
-
D
Specified equation of state from heat capacity
Homework Statement The constant-volume heat capacity of a particular simple system is c_v = AT^3 where A is a constant. In addition the equation of state is known to be of the form (v-v_0)p = B(T) where B(T) is an unspecified function of T. Evaluate the permissible functional form of B(T)...- Dazed&Confused
- Thread
- Replies: 4
- Forum: Advanced Physics Homework Help
-
D
Compresibility of helium gas
Homework Statement A cylinder is fitted with a piston, and the cylinder contains helium gas. The sides of the cylinder are adiabatic, impermeable, and rigid, but the bottom of the cylinder is thermally conductive, permeable to helium, and rigid. Through this permeable wall the system is in...- Dazed&Confused
- Thread
- Replies: 7
- Forum: Advanced Physics Homework Help
-
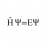
Finding compressibility from given internal Energy function
Hi everyone! 1. Homework Statement Given is a function for the internal energy: ##U(T,V)=Vu(T)## Asked is to derive the entropy balance equation. In order to do so i need to find the "isothermal and adiabatic compressibility": $$\kappa_{T}=-\frac{1}{V}\left(\frac{\partial V}{\partial...- H Psi equal E Psi
- Thread
- Replies: 7
- Forum: Advanced Physics Homework Help
-
F
Thermodynamics - isentropic, polytropic and compressibility
Several questions: What does compression actually means? In the case of isentropic calculations a changed in density (or specific volume) is included in the calculations (isentropic.jpg) so isentropic means that compressibility is included in those calculations or what?In a previous thread, a...- Friis
- Thread
- Replies: 1
- Forum: Thermodynamics
-
L
Thermodynamics Compressibility Factor
Homework Statement Please consider ethylene at 152oF and 126 atm. Please determine the molar volume (ft3/lbmole) if Z is determined by Corresponding States Theory. Homework Equations Z=PVm/RmT Vm= Molar volume R=Rm/M M= molecular weight Rm=1545(ft*lbf)/(lbmol*oR) Zc=(Pcvc)/(R*Tc) Tc=283 K...- Logan McEntire
- Thread
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-

Compressibility factor for an Isotherm
Homework Statement Why is it that when considering the compressibility factor of a gas Z we see the value decrease at first and increase as value for Pr increases for an isotherm Tr=1? Homework Equations z=1+(Pb/RT)-(a/VRT) The Attempt at a Solution It is my understanding that as Pr is low...- HethensEnd25
- Thread
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
F
I Compressibility of liquid vs solid actual numbers
I was trying to find the compressibility of water and compressibility of air to compare. For compressibility of water I found 46.4e-6 For compressibility of a gas... I am having a tough time finding anything. compressibility factor I can find, which is 1 for Hydrogen... but how does that relate...- fahraynk
- Thread
- Replies: 9
- Forum: Other Physics Topics
-
Z
I How do you find Z when the temperature line ends on the compressibility chart?
How do you find a value for Z using the compressibility chart when the temperature line you're trying to use ends? For example, if you look at the compressibility chart and try to find a value for z with P_r = .75 and T_r = .96, you will notice that the line for T_r = .95 ends well before the...- zachdr1
- Thread
- Replies: 7
- Forum: Other Physics Topics
-

Isothermal Compressibility of Photon Gas
I am really stuck at this question. I tried to get the equation of volume with independent variables P and T, but the equation itself does not give a nice form, and thus I cannot get the derivative of V with respect to P. What should I do?- fricke
- Thread
- Replies: 1
- Forum: Thermodynamics
-
J
Isothermal Compressibility: Derive an equation
> The isothermal compressibility $\kappa_t$ of a substance is defined as $$ \kappa_t = -\frac{1}{V} \left ( \frac{\partial V}{\partial P} \right )_T $$ Obtain an expression for the isothermal compressibility of an ideal gas. (PV = RT) in terms of p. I believe that the ideal gas law equation...- johnr
- Thread
- Replies: 2
- Forum: Advanced Physics Homework Help
-
G
How Much Water is Needed to Increase Pipeline Pressure for a Hydro Test?
I am trying to figure if there is a calculation for working out how much water I would have to pump into a system to increase the pressure. For example, I have a pipeline which is 2" and 6.5 km long. I worked the volume out using πr² × height so π×0.0254m²×6500m which is 13.17m³. How do I now...- G Neilson
- Thread
-
- Tags
- Compressibility Volume Water
- Replies: 4
- Forum: Mechanics
-
P
Heating of liquid when pressure 1bar-->5000bars
Hello! I have some troubles finding a way to estimate how much temperature of liquid would rise when it is suddenly adiabatically pressurized to thousands of bars. In normal conditions liduids such as water are considered to be incompressible, but certainly not in 5000 bars. Because water (or...- Paavo Palikka
- Thread
- Replies: 5
- Forum: Thermodynamics
-
A
Chorin Artificial Compressibility Equations
Hi! I have the following problem: pt + (c2u)x + (c2v)y = 0 ut + (u2+p)x + (uv)y = α(uxx+uyy) vt + (uv)x + (v2+p)y = α(vxx+vyy) It is a formulation of the incompressible Navier-Stokes equations. I would like to know an exact solution. Can anyone help me? Thanks- angy
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
A
What does "compressibility is abridged" translate to?
I'm doing an independent research in aeronautics (realistically I'm just learning the material on my own and testing out of it due to unforeseen circumstances last semester). One area of study that my professor advised me to research in depth was just this simple phrase "compressibility is...- air_novice
- Thread
-
- Tags
- Compressibility
- Replies: 1
- Forum: Mechanical Engineering
-
S
Compressibility factor and van der Waals equation for temp
The pressure exerted on the walls of the container by a real gas is less compared to an ideal gas. This is due to the attractive forces of the gas pulling the molecules back towards the rest of the gas molecules. However, there is also a relationship whereby at lower temperatures, the z is even...- sgstudent
- Thread
- Replies: 3
- Forum: Classical Physics
-
S
Question about compressibility factor
At low temperatures, z falls below 1 and the reason for that is because the intermolecular interactions cause the pressure exerted to be lesser than expected. PVm/RT=z and since P is less than expected z drops below 1. However, as the pressure increases z increases to be above 1 because as P...- sgstudent
- Thread
- Replies: 1
- Forum: Other Physics Topics
-
C
Work and isothermal compressibility
Homework Statement 1 kg of water is at room temperature and the pressure is isothermally increased on the system from 1 atmosphere to 1000 atmospheres. What is the work done? What is the change in heat? What would be the temperature change if this was done adiabatically? The volumetric...- Chris B
- Thread
- Replies: 8
- Forum: Introductory Physics Homework Help
-
M
Equations of state -- Partial derivatives & Expansivity
Homework Statement Show that the coefficient of volume expansion can be expressed as β= -1÷ρ (∂ρ÷∂T) keeping P (pressure) constant Where rho is the density T is Temperature Homework Equations 1/v =ρ β= 1/v (∂v÷∂T) keeping P (pressure ) constant The Attempt at a Solution I started with...- Mia_S
- Thread
- Replies: 7
- Forum: Advanced Physics Homework Help
-
A
Finding vapour pressure using compressibility chart
Homework Statement I was asked to find the vapour pressure and saturated liquid molar density of propane at 263.15K using a generalised compressibility chart. (not allowed to use NIST or steam tables either, the chart i was given does not have reduced volume lines) Homework Equations Tr=T/Tc...- annnoyyying
- Thread
- Replies: 14
- Forum: Engineering and Comp Sci Homework Help
-
Z
How to Calculate Isothermal Compressibility for Question 6b?
Homework Statement Homework Equations No idea... The Attempt at a Solution Does anyone know how to do question 6b)? Its a past paper question for an exam in 2 days and i have literally no idea where to start.- z_abideen
- Thread
- Replies: 6
- Forum: Engineering and Comp Sci Homework Help
-
M
Solving an Equation of State Using Compressibility & Expansivity
Homework Statement Obtaining an equation of state from compressibility and expansivity. States of superheated steam are observed to have an isothermal compressibility k=(rNT) / (VP^2) and a volume expansivity B=(N/V)((r/P)+(am / T^m+1)). r,m and a are constants. a) Find dv in terms of dP...- Mic :)
- Thread
- Replies: 27
- Forum: Calculus and Beyond Homework Help
-

Linear compressibility Lennard-Jones potential
Homework Statement Please see attachment. The correct answer is supposed to be ##\alpha = .15 k_B/U_0##. The Attempt at a Solution The minimum of the potential is at ##x = a##. Expanded about this minimum we get ##U = -U_0 + 36\frac{U_0}{a^2}(x - a)^2 - 252\frac{U_0}{a^3}(x - a)^3 + ...##...- WannabeNewton
- Thread
- Replies: 16
- Forum: Advanced Physics Homework Help
-

Isothermal compressibility and spring constant solids
Homework Statement Consider a solid of compressibility ##\kappa##. Assume that the atoms in this solid are arranged on a regular cubic lattice, the distance between their nearest neighbors being ##a##. Assume further that a restoring force ##-k_0 \Delta a## acts on a given atom when it is...- PhizKid
- Thread
- Replies: 5
- Forum: Introductory Physics Homework Help
-
G
Thought experiment about fluid compressibility
I'm a physics noob but this is something I was thinking about when I read in my textbook that fluids are generally incompressible. Let's say I had 1000 L of water enclosed in a sphere several meters thick (say, 2 meters thick) made of an extremely hard, dense metal with 0 outlets or holes of...- grayb
- Thread
- Replies: 5
- Forum: Classical Physics
-
A
Compressibility Factor Pressure For Calculating Fan Power
What is the correct type of pressure (static or total) used in the compressibility factor, KP, when calculating fan power? Howden's Fan Engineering book seems to indicate total pressures should be used, but I also have a PDF from Howden that indicates static pressures should be used. Online...- Aperture Labs
- Thread
- Replies: 3
- Forum: Mechanical Engineering
-
B
Finding out compressibility of a fluid when bubbles are present?
I want to know approximately how compressible a fluid can become when H2O bubbles are present. Does anyone know how to do this? Thanks.- bzz77
- Thread
-
- Tags
- Bubbles Compressibility Fluid
- Replies: 6
- Forum: General Engineering
-
M
Volume using Compressibility Factor
Homework Statement Find the volume of 2 kg of ethylene at 270 K, 2500 kPa using Z Homework Equations Method to Solve for Z, Using Tr and Pr PV = ZnRT The Attempt at a Solution Tr = 0.9561 Pr = 0.496 Z found to be approx. 0.75 R given on a table at 0.2964 kJ/kg K From...- MechE2015
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
O
Prove expansibility / isothermal compressibility
β is expansibility , (1/V)(∂V/∂T) at constant temperature κ is isothermal compressibility , (-1/V)(∂V/∂P) at constant pressure How to prove (∂P/∂T) at constant V = (β/κ) Thank you- Outrageous
- Thread
- Replies: 2
- Forum: Thermodynamics
-
A
Finding the compressibility factor (Z)
Homework Statement Determine the volume, in m^3, occupied by 20 kg of hydrogen (H2) at 1170 kPa, 2220°C. Homework Equations Z=pv/rt, Pr=P/Pc, Tr=T/Tc, and for hydrogen M = 2.016 (kg/kmol) Tc = 33.2 (K) Pc = 13.0 bar Zc=pc*vc/(RTc) The Attempt at a Solution I know if I find Z then...- awyea
- Thread
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
G
Isothermal compressibility of bose-Einstein condensate
Homework Statement Variables: N (number of particles), μ (chemical potential), P (pression), V (volume). k is Boltzmann's constant. I often use β=1/kT. The (isothermal) compressibility is given by \kappa_{T} = -\frac{1}{V}\left (\frac{\partial V}{\partial P}\right )_{N,T} The...- gbertoli
- Thread
- Replies: 4
- Forum: Advanced Physics Homework Help
-
B
Help with Compressibility of liquid and relation to pressure
Homework Statement Assume that the distance across a microscopic cell is larger than the correlation length of the liquid, so whatever is happening in one cell is statistically uncorrelated with what is happening in an adjacent cell. Further, assume that each cell has two distinct possible...- bearit88
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
S
Is Infinite Compressibility Possible in a System?
Correct me if I am wrong, but I think that compressibility, generally tells how difficult it is to compress an object. Meaning, the higher the compressibility, the easier it will be to compress an object with a given pressure. I was asked in my University to describe an system of infinite...- ShadowDatsas
- Thread
-
- Tags
- Compressibility Infinite
- Replies: 4
- Forum: Classical Physics
-
N
Compressibility of Ice vs Water: Answers & Explanations
Dear PF, Is ice compressible? What its compressibility... or compressibility relatively to water compressibility Thanks much- Neitrino
- Thread
-
- Tags
- Compressibility Ice
- Replies: 2
- Forum: Other Physics Topics
-

Water density and compressibility
Water is compressible, but it takes a lot of pressure to increase the density by 10 or 20% Deep Ocean pressure measurements http://nctr.pmel.noaa.gov/Dart/Pdf/Eble_J_atmo_91.pdf http://seagrant.gso.uri.edu/factsheets/abyss.html http://www.windows2universe.org/earth/Water/density.html...- Astronuc
- Thread
-
- Tags
- Compressibility Density Water
- Replies: 2
- Forum: Earth Sciences
-
O
Finding coefficient of thermal expansion and isothermal compressibility
Homework Statement To a very good approximation, ammonia obeys the Bertholet equation of state, which readsPV=nRT+\frac{9}{128}(\frac{nRTc}{Pc})(1-6\frac{Tc^2}{T^2})Pa)Suppose we have 500 grams of ammonia under a pressure of P=3.04 atm and at T=323K. Calculate the volume of ammonia according...- osker246
- Thread
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
T
Increase in compressibility when pressure is applied
I was running some structural relaxations under constant pressure, and found out that my system has compressibility which increases when pressure is applied. Of course, this is only up to a point, since at pressure high enough, phase transition will occur and compressibility will drop sharply...- tomkeus
- Thread
- Replies: 1
- Forum: Atomic and Condensed Matter
-
H
An example of a fluid with low compressibility
Hi, Does anyone know a fluid with low compressibility? Thanks, H- hoomanya
- Thread
-
- Tags
- Compressibility Example Fluid
- Replies: 4
- Forum: Mechanical Engineering
-
R
Real gas compressibility isotherms(graphs)
Homework Statement Sketch the following compressibility isotherms(fancy word for graphs) (compressibility factor, PV/nRT versus pressure :) for oxygen at a temperature just above the critical temperature ::) for oxygen at a temperature well above the critical temperature eg. 200 degrees...- requal
- Thread
-
- Tags
- Compressibility Gas Real gas
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
S
Isothermal Compressibility always positive proof
I have a question on the quantity -(dV/dP)T,N where V = volume, P = pressure, T = temp, N = number of moles and T, N are held constant. I see in textbooks that this quantity is always positive at equilibrium. It makes intuitive sense, as if it were negative, it would be unphysical. I've been...- skateboarding
- Thread
- Replies: 4
- Forum: Thermodynamics
-

Compressibility of a Refrigerant
I am going to be running some experiments with refrigerants. They will be at various temperatures and pressures. I will then be doing some modeling of certain phenomena. I would like to be able to quantify whether the ideal gas model is reasonable during certain stages of the experiment. My...- Saladsamurai
- Thread
- Replies: 1
- Forum: Mechanical Engineering
-
G
Compressibility Factors of Gas
Homework Statement Calcualte the volumes in cubic feet of the gases in the attached chart, assuming the gases are ideal Homework Equations Ideal gas equation PV=nRT The Attempt at a Solution Please see the attached sheet also, this has my attempt, if i use my method and then use a...- glocki35
- Thread
-
- Tags
- Compressibility Factors Gas
- Replies: 1
- Forum: Engineering and Comp Sci Homework Help
-
J
[Thermo.] thermal expansion and isothermal compressibility coefficients.
Homework Statement A certain metal whose thermal expansion coefficient \beta is 5,0 × 10^-5 °C^-1 and whose isothermal compressibility \kappa_T is 1,2 × 10^-6 atm^-1 is at an initial pressure of 1 atm and an initial temperature of 20°C. A thick layer of Invar is thermally insulating the...- Je m'appelle
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
M
Adiabatic Compressibility
I have been asked to show that if an ideal gas is compressed isothermally its compressibility is 1/P whereas if the same gas is compressed adiabatically its compressibility is 1/yP Where y is gamma I have managed to do the first bit about isothermal compressibility, but cannot work out...- Milly_S
- Thread
-
- Tags
- Adiabatic Compressibility
- Replies: 1
- Forum: Advanced Physics Homework Help