- #1
Dant_li
- 1
- 1
- Homework Statement
- Hello, I’m in high school (junior) and I was reading this book in nuclear physics and they mention alpha decay and I search up and I found this photo with branching ratios. I get the concept itself. But I was wondering if someone could explain to me the branching ratios on the photo as well as why there’s no decay to ground state of 237Pu?
- Relevant Equations
- BR = ki/(k1+k2+....ki...+) = ki/k
I understand that In general, the branching ratio
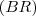 for a particular decay mode is defined as the ratio of the number of atoms decaying by that decay mode to the number decaying in total. But I can’t get this specific branching ratio.
for a particular decay mode is defined as the ratio of the number of atoms decaying by that decay mode to the number decaying in total. But I can’t get this specific branching ratio.
