- #1
Karim Habashy
- 33
- 1
Hi All,
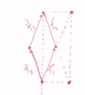
Using (Mirror + Translation in 2D).
I see that Diamond and Center Rectangular Lattice in 2D has the same Symmetry i.e:
1)Horizontal Mirror Plane
2)Vertical Mirror Plane
3)2-Fold Rotation Axis.
and Diamond has Smaller Area.
Then why we say Center Rectangular has higher Symmetry and we take it as the Standard Cell.
Image Attached.
Reference, Edx.org, Course:
Symmetry, Structure and Tensor Properties of Materials, Lecture 7.
Thanks.
Using (Mirror + Translation in 2D).
I see that Diamond and Center Rectangular Lattice in 2D has the same Symmetry i.e:
1)Horizontal Mirror Plane
2)Vertical Mirror Plane
3)2-Fold Rotation Axis.
and Diamond has Smaller Area.
Then why we say Center Rectangular has higher Symmetry and we take it as the Standard Cell.
Image Attached.
Reference, Edx.org, Course:
Symmetry, Structure and Tensor Properties of Materials, Lecture 7.
Thanks.