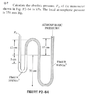
- #1
TyErd
- 299
- 0
Homework Statement
the question is in the diagram i have attached. basically i have to find absolute pressure
Homework Equations
P = pgh where P is Pressure and p is density
The Attempt at a Solution
1st of all i need to convert 750mm into a pressure? somehow don't know exactly.
the rule i was told is starting from the right side, if it goes down it is subtracted and if it goes up it is added. So in knowing that i did: p_atm - (8 * 0.15 ) - ( 10* 0.05) but i don't know the pressure yet coz i don't know how to calculate. is the rest of the method correct though?