- #1
Taniaz
- 364
- 1
Homework Statement
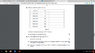
Given values and uncertainties of d, find the absolute uncertainty in 1/d^2 (see table attached)
Homework Equations
I know that if it's a square function the absolute uncertainty will be 2 (delta d) / d but I'm not sure if it's correct for an inverse function.
The Attempt at a Solution
I read somewhere that it might be 2 (deIta d)/ d^3? If I use this equation, I get the range of answers they gave in the marking scheme which are 0.03 till 1. If I use only 2(delta d)/d I get uncertainties from 0.02 till 0.07.
Thank you