- #1
zorro
- 1,384
- 0
Homework Statement
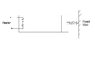
An ideal monoatomic gas is confined in a cylinder by a spring-loaded piston of cross section 8 x 10-3m2. Initially the gas is at 300K and occupies a volume of 2.4 x 10-3 m3 and the spring is in its relaxed state. The gas is heated by a smalll heater until the piston moves out slowly by 0.1 m. Calculate the final temp of the gas. The force constant of the spring equals 8000N/m, Patm = 1atm. The cylinder and the piston are thermally insulated. The piston and the spring are massless and there is no friction between the piston and the cylinder. Neglect any heat loss through lead wires of the heater. The heat capacity of the heater coil is negligible.
The Attempt at a Solution
Work done by the gas in expansion
W = 0.5kx2 + Patm x A x 0.1
Since the cylinder and piston are thermally insulated.
Work done equals the change in internal energy i.e. W=nCv[tex]\Delta[/tex]T
on solving, nCv[tex]\Delta[/tex]T = 120
now Cv=1.5R and n=PV/RT
so 1.5PV[tex]\Delta[/tex]T/T = 120
P,V,T are known to us
so [tex]\Delta[/tex]T = 100
T2 - T=100
T2=100 + 300 =400
But the answer is 800 K which only differs by a factor of 2.
Please tell me what is the problem.
I checked all my calculations. They are correct.