- #1
davidwinth
- 101
- 8
- TL;DR Summary
- If the transmittance of IR to space is already zero in CO2 bands, how can adding more CO2 change anything?
This is a serious question, and it is not meant as an attempt to "debunk" greenhouse warming of the earth.
Wikipedia has the below image, which shows the atmospheric transmittance as a function of wavelength. Notice the red vertical line through one of the CO2 absorption bands. We see that the light emitted in that band is nearly totally absorbed by the CO2 in the atmosphere, i.e., the transmittance is near zero in that band (and the same for the thinner band at shorter wavelength). Now the question arises: how could adding more CO2 make it so that even more light is absorbed in that band, when all the IR emitted in that band is already absorbed by CO2?
My understanding is that the AGW hypothesis is that IR emitted from the ground is absorbed by CO2 in the atmosphere and this prevents it from getting to space and thereby cooling the earth. If this is so, then isn't the atmosphere already at a point where it cannot absorb more (than 100%) of the light in that band?
Thank you!
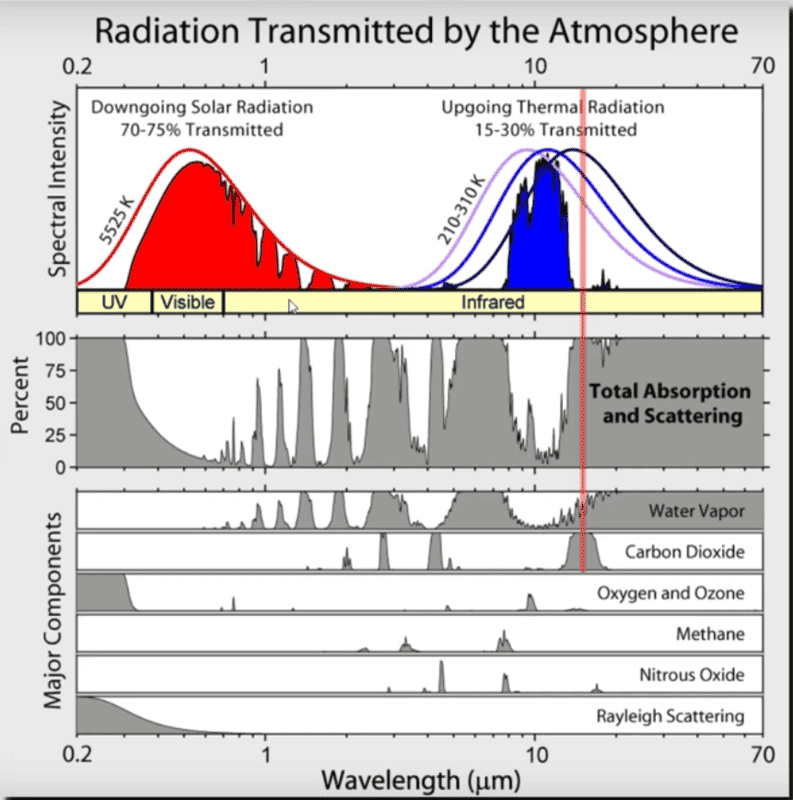
Wikipedia has the below image, which shows the atmospheric transmittance as a function of wavelength. Notice the red vertical line through one of the CO2 absorption bands. We see that the light emitted in that band is nearly totally absorbed by the CO2 in the atmosphere, i.e., the transmittance is near zero in that band (and the same for the thinner band at shorter wavelength). Now the question arises: how could adding more CO2 make it so that even more light is absorbed in that band, when all the IR emitted in that band is already absorbed by CO2?
My understanding is that the AGW hypothesis is that IR emitted from the ground is absorbed by CO2 in the atmosphere and this prevents it from getting to space and thereby cooling the earth. If this is so, then isn't the atmosphere already at a point where it cannot absorb more (than 100%) of the light in that band?
Thank you!
Last edited: