- #1
scientifico
- 181
- 0
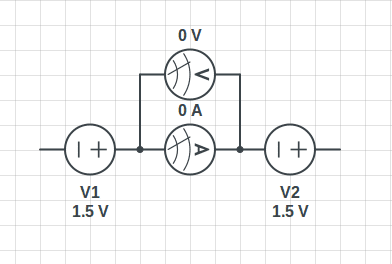
Hello, I can't image what is the exact path of electrons in two or more batteries conncted in series... if electrodes are immersed into the electrolytes and chemical reactions should continously happen why the circuit in the picture down here has no activity ?