- #1
otaKu
- 27
- 2
This website here says that the expression for binding energy for an electron is:
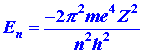 This http://ocw.mit.edu/high-school/chemistry/exam-prep/structure-of-matter/atomic-theory-and-atomic-structure/MITHFH_lecnotes05.pdfby MIT calculates it quantum mechanically to give:
This http://ocw.mit.edu/high-school/chemistry/exam-prep/structure-of-matter/atomic-theory-and-atomic-structure/MITHFH_lecnotes05.pdfby MIT calculates it quantum mechanically to give:
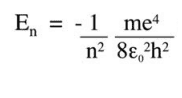 The book I was reading optoelectronics from says that the energy binding the electron to the impurity(ionic nucleus) is
The book I was reading optoelectronics from says that the energy binding the electron to the impurity(ionic nucleus) is
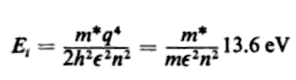 I am totally confused as to why there is a discrepancy between these results. Don't they mean the same(not talking about the inclusion of atomic number in first and effective mass in third)? Since all of these denote the energy of the electron bound to the core(nucleus) why do they differ?! Am I overlooking something and all three of these are correct? I would highly value any advice or explanation on this discrepancy. I've referred multiple sources and feel completely lost even though it is something very fundamental and basic. Thank you!
I am totally confused as to why there is a discrepancy between these results. Don't they mean the same(not talking about the inclusion of atomic number in first and effective mass in third)? Since all of these denote the energy of the electron bound to the core(nucleus) why do they differ?! Am I overlooking something and all three of these are correct? I would highly value any advice or explanation on this discrepancy. I've referred multiple sources and feel completely lost even though it is something very fundamental and basic. Thank you!
(Follow the links to see the sources for the equations)
(Follow the links to see the sources for the equations)
