- #1
Stephanus
- 1,316
- 104
Dear PF Forum,
I'm trying to understand antioxidant and free radicals. But I'm afraid that my chemistry is weak.
Perhaps someone can help me with this picture?
https://en.wikipedia.org/wiki/Hydrogen_peroxide
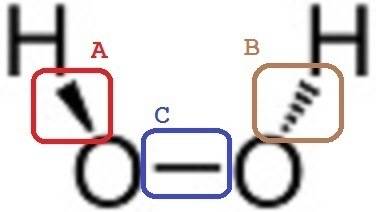
This is Hydrogen Peroxide. Neutral.
As much as I can surmise in this picture is...
1. It's H2O2, I don't need the picture to guess H2O2, Hydrogen Peroxide is clear.
2. Oxygen (Z = 8, electron configuration: 1s2 2s2 2s4 , Oxygen lacks 2 electrons in its outer shell.
3. Hydrogen (Z = 1, 1s1 ), lacks 1 electron
4. Left Oxygen is bound to upper left Hydrogen and right Oxygen, making it complete.
5. So is Right Oxygen.
6. Upper left Hydrogen is bound to left Oxygen, making it complete
7. So is Right Hydrogen.
What I want to ask is that symbol
A: Triangle
B: Broken triangle
C: Rectangle
Why A, B and C pictures are different? Are they bonded in different ways?
Thanks for any help.
I'm trying to understand antioxidant and free radicals. But I'm afraid that my chemistry is weak.
Perhaps someone can help me with this picture?
https://en.wikipedia.org/wiki/Hydrogen_peroxide
This is Hydrogen Peroxide. Neutral.
As much as I can surmise in this picture is...
1. It's H2O2, I don't need the picture to guess H2O2, Hydrogen Peroxide is clear.
2. Oxygen (Z = 8, electron configuration: 1s2 2s2 2s4 , Oxygen lacks 2 electrons in its outer shell.
3. Hydrogen (Z = 1, 1s1 ), lacks 1 electron
4. Left Oxygen is bound to upper left Hydrogen and right Oxygen, making it complete.
5. So is Right Oxygen.
6. Upper left Hydrogen is bound to left Oxygen, making it complete
7. So is Right Hydrogen.
What I want to ask is that symbol
A: Triangle
B: Broken triangle
C: Rectangle
Why A, B and C pictures are different? Are they bonded in different ways?
Thanks for any help.