- #1
williamcarter
- 153
- 4
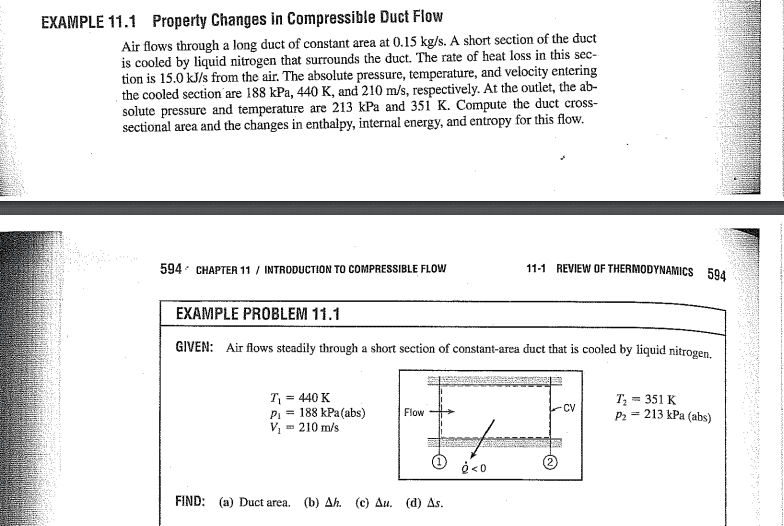
Hello, I tried attempting the following example problem 11.1(please see below), however I don't quite get from where they got cp=1.00 and cv=0.717?
I know that Q=m*cp*delta T=> cp=Q/(m*delta T)
Q=n*cv*delta T=>cv=Q/(n*deltaT)
Problem statement
 Their solution:
Their solution:
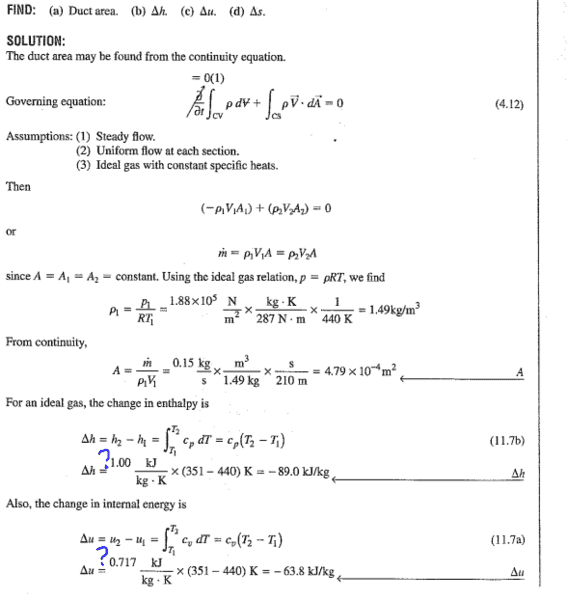
How did they manage to get for cp=1.00 and cv=0.717?
Thank you
I know that Q=m*cp*delta T=> cp=Q/(m*delta T)
Q=n*cv*delta T=>cv=Q/(n*deltaT)
Problem statement
How did they manage to get for cp=1.00 and cv=0.717?
Thank you