- #1
robertjford80
- 388
- 0
Homework Statement
The Attempt at a Solution
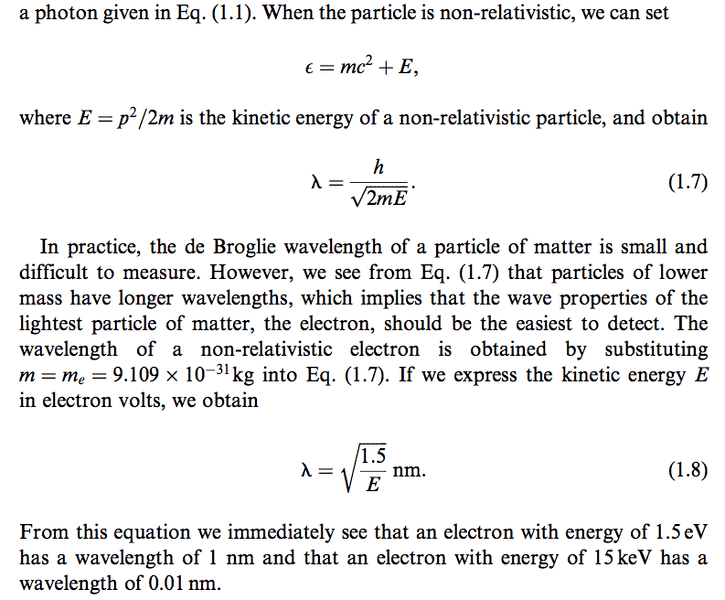
I don't see how they got sqrt(1.5/E) I tried it like this
Not the right answer.
robertjford80 said:Homework Statement
The Attempt at a Solution
I don't see how they got sqrt(1.5/E) I tried it like this
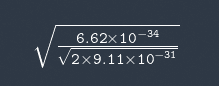
Not the right answer.
The De Broglie wavelength is a concept in quantum mechanics that describes the wavelength of a particle, such as an electron, based on its momentum. It is named after French physicist Louis de Broglie.
The De Broglie wavelength is calculated using the formula λ = h / p, where λ is the wavelength, h is Planck's constant, and p is the momentum of the particle.
The De Broglie wavelength is significant because it shows that particles, such as electrons, have both wave-like and particle-like properties. This helped to shape our understanding of quantum mechanics and the behavior of subatomic particles.
The De Broglie wavelength is affected by the mass and velocity of the particle. As the mass increases, the wavelength decreases, and as the velocity increases, the wavelength also decreases.
The De Broglie wavelength is related to the uncertainty principle, which states that the more precisely we know the momentum of a particle, the less precisely we can know its position. This is because measuring the momentum of a particle requires interacting with it, which can change its position. The De Broglie wavelength is a manifestation of this principle.